Resources
About Us
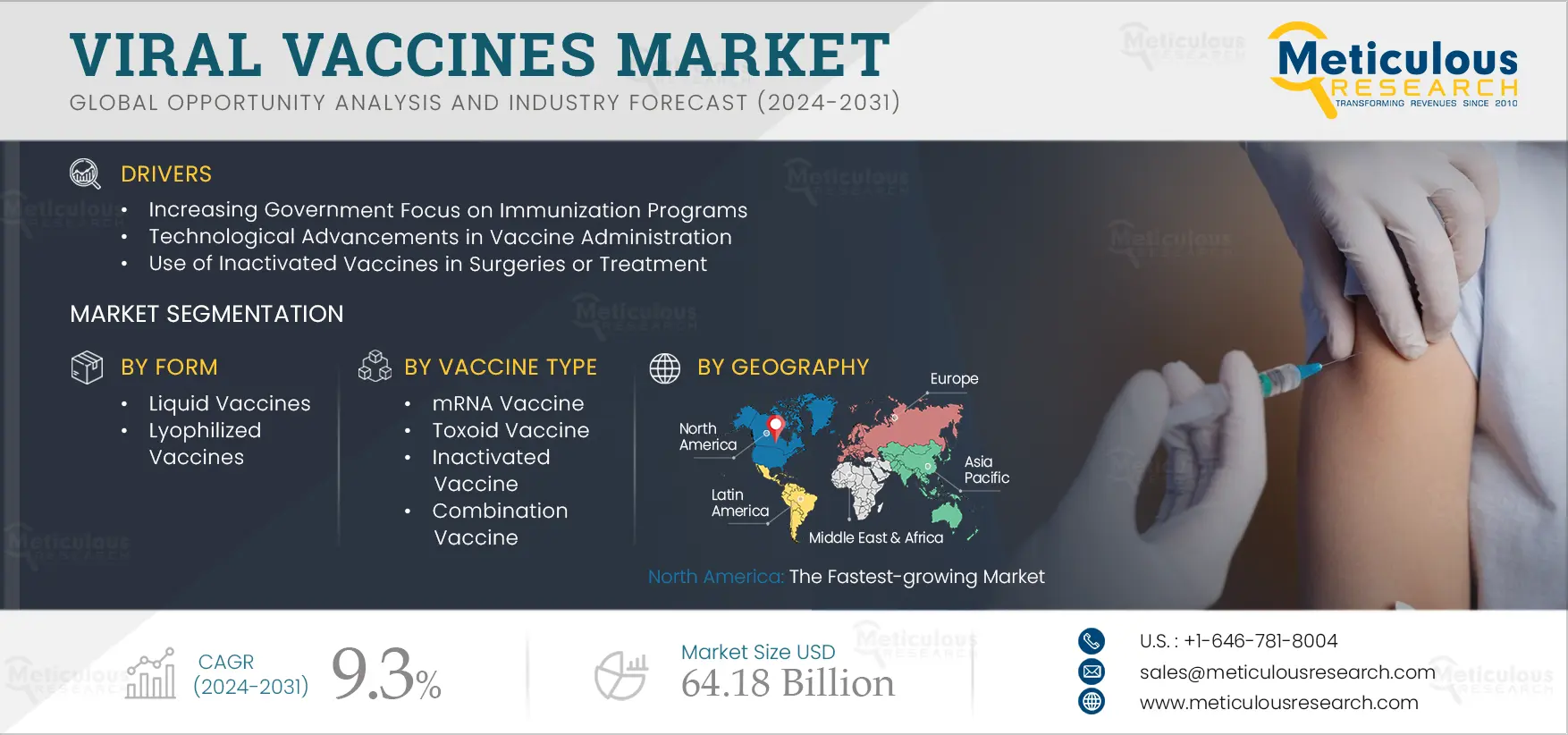
Viral Vaccines Market Size, Share, Forecast, & Trends Analysis by Form (Liquid, Lyophilized) Type (Live Attenuated, mRNA, Inactivated), Route of Administration (Intramuscular, Subcutaneous, Oral), Approach, Indication, Packaging – Global Forecast to 2031
Report ID: MRHC - 1041237 Pages: 239 Nov-2024 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportThe growth of this market is driven by the increasing government focus on immunization programs, technological advances in vaccine administration, and the use of inactivated vaccines in surgeries and treatments. However, the high costs of vaccine development and the long timelines of vaccine manufacturing restrain the growth of this market.
Furthermore, the growing focus on therapeutic vaccines, this increasing use of adjuvants in vaccines, growth prospects in emerging markets, and the development of highly stable and energy-efficient ultra-low-temperature freezers are expected to generate growth opportunities for market stakeholders. However, product recalls and inadequate access to vaccines are major challenges impacting the market’s growth.
Vaccine technology has evolved significantly during the last decade, profoundly changing the future of vaccine development. The introduction of genetic engineering has fuelled many advances in vaccine development programs, leading to new products. Various virus vaccines that use the attenuated form of the virus have been developed through these methods. Also, new technologies enable the faster detection of viruses and allow for concentration levels to be kept high enough to generate an immune response.
Recent vaccine technologies are largely driven by the need to accelerate response times against emerging threats and make vaccines available for quick deployment. The increasing need to develop vaccines against difficult targets and improve delivery systems for maximum potency is also the focus of innovation in the vaccine industry. The development of synthetic vaccine candidates, genomic analysis of disease progression and vaccine response, structure-based antigen design, and nanoparticle delivery systems are a few of the technological advancements in vaccine delivery. Therefore, technological advancements are expected to drive the emergence of new and more effective vaccines for various new indications, fuelling the growth of the viral vaccines market.
 Click here to: Get a Free Sample Copy of this report
Click here to: Get a Free Sample Copy of this report
Historically, vaccines have been used primarily to prevent diseases, but a new category known as therapeutic vaccines has recently emerged. Therapeutic vaccines are designed to treat diseases by inducing or enhancing the body's immune response. Unlike preventive vaccines, which aim to prevent disease before it occurs, therapeutic vaccines target patients who are already affected by the disease, helping to treat or manage the condition. Since the success of the first therapeutic vaccine post-2010, key players in the vaccine industry have increasingly focused on developing therapeutic vaccines for various conditions, particularly cancers. For instance, as of May 2024, Pfizer Inc. (U.S.) has 17 vaccines in its pipeline, targeting diseases and infections such as influenza, hepatitis, Lyme disease, invasive group B streptococcus, and respiratory syncytial virus (RSV), among others.
Although therapeutic vaccines currently represent a small market—mainly limited to vaccines for skin, prostate, and bladder cancers, as well as a few for pollen allergies—this market is expected to expand significantly in the future. With growth projections outpacing those for preventive vaccines, therapeutic vaccines offer opportunities for manufacturers in the coming years.
Personalized vaccines represent the latest advancement in immunization, designed to elicit immune responses against specific antigens and microorganisms tailored to an individual’s condition. These vaccines aim to establish long-lasting immunity while minimizing side effects. By customizing the vaccine to the patient's unique needs, personalized vaccines can improve safety profiles and reduce reactogenicity. Additionally, viral vectors used in these vaccines can be genetically modified to make them replication-deficient, further enhancing their safety.
Various personalized vaccines are being developed to target diseases, particularly different types of cancer. For instance, in April 2024, researchers from the American Association for Cancer Research (AACR) reported the development of a new therapeutic cancer vaccine, TG4050. This vaccine targets head and neck squamous cell carcinoma (HNSCC) and is based on an engineered form of the vaccinia virus. TG4050 delivers 30 personalized neoantigens that are unique to each patient's tumor, stimulating the activation of an antitumor immune response.
Public awareness plays a crucial role in sensitizing communities about the importance of vaccines and vaccination. Immunization is the process by which a person is protected from disease through vaccination. It is one of the most successful and cost-effective public health interventions, saving millions of lives each year. According to the World Health Organization (WHO), immunization prevents approximately 4-5 million deaths annually from infectious diseases such as hepatitis, tetanus, measles, and polio.
However, globally, around 1.15 million children in the Western Pacific Region miss out on essential vaccinations. In order to address this, governments and health organizations are organizing immunization programs to ensure that all individuals, including those in rural areas, receive vaccinations. For example, in December 2022, the National Technical Advisory Group on Immunization (NTAGI) in India recommended the introduction of the human papillomavirus (HPV) vaccine into the Universal Immunization Programme (UIP), with a one-time catch-up for girls aged 9-14, followed by routine vaccination for 9-year-olds.
Therapeutic vaccines are generally administered after a disease or infection has affected the patient and they work by activating the immune system of a patient. Therapeutic vaccines are also being majorly used as a viable option for active immunotherapy of cancers. Unlike preventive vaccines, therapeutic vaccines can be customized to respond to specific mutations. These vaccines differ from traditional vaccines as they target the pathogens directly instead of producing antibodies and generating immune proteins. Apart from cancers, therapeutic vaccines can also be used to treat infections due to the human papillomavirus (HPV).
Recent times have witnessed remarkable developments and advancements in vaccine technologies to improve immunization in patients. Certain market players are also collaborating to develop new therapeutics for cancer treatment. For instance, in April 2024, CureVac SE (Germany) collaborated with the University of Texas MD Anderson Cancer Center (U.S.) to develop novel mRNA-based cancer vaccines.
Based on forms, the market is segmented into liquid vaccines and lyophilized vaccines. In 2024, the liquid vaccines segment is expected to account for the larger share of 78.9% of the viral vaccines market. Liquid viral vaccines are formulations containing live or weakened viruses, which serve as the primary component to induce an immune response in the body. These vaccines are typically administered via injection. The large share of this segment is attributed to factors such as ease of administration, reduced risk of errors, decreased wastage, improved patient experience, lower production costs, and enhanced stability. For instance, Bharat Biotech Ltd. (India), a manufacturer of vaccines and bio-therapeutics, offers a live attenuated rotavirus vaccine in its product portfolio. This liquid frozen vaccine contains the live rotavirus 116E strain and is used to prevent rotavirus gastroenteritis.
Based on vaccine types, the market is segmented into subunit & conjugate vaccines, live attenuated vaccines, inactivated vaccines, mRNA vaccines, viral vector vaccines, toxoid vaccines, and combination vaccines. In 2024, the mRNA vaccines segment is expected to account for the largest share of 63.1% of the viral vaccines market. mRNA vaccines are a type of genetic vaccine that uses messenger RNA (mRNA) to instruct cells to produce proteins that trigger an immune response against a specific virus. The large market share of this segment is attributed to several factors, including the superior stability of mRNA vaccines compared to other types, an effective delivery system, and the use of lipid nanoparticles, which help deliver the mRNA to cells by facilitating its attachment to the cell membrane.
Based on approaches, the market is segmented into preventive vaccines and therapeutic vaccines. In 2024, the preventive vaccines segment is expected to account for the larger share of the viral vaccines market. Preventive (or prophylactic) vaccines are designed to prevent diseases before exposure to pathogens. These vaccines stimulate the immune system to recognize and combat infectious agents, helping reduce the incidence and severity of diseases. The large market share of this segment is driven by factors such as the increasing prevalence of chronic and infectious diseases, rising public awareness about immunization, growing immunization programs, the induction of herd immunity, and the prevention of disease outbreaks. For instance, in April 2024, the Pan American Health Organization (PAHO) launched the 22nd edition of Vaccination Week in the Americas (VWA) 2024, which included updates on vaccination coverage and efforts to return to pre-pandemic vaccination levels.
However, the therapeutic vaccines segment is expected to grow at the fastest CAGR during the forecast period. Therapeutic vaccines are administered to individuals already suffering from infections or diseases to help reduce symptoms and modify the course of the disease. These vaccines are primarily used to treat certain types of cancer. The growth of this segment is driven by factors such as the introduction of antiviral immunity to alter disease progression, high specificity and safety, low susceptibility to side effects, and cost-effectiveness.
Based on indications, the market is segmented into influenza, human papilloma virus (HPV), rotavirus, poliomyelitis (polio), hepatitis, cancer, and other indications. In 2024, the influenza segment is expected to account for the largest share of 40% of the market. The large market share of this segment is attributed to factors such as increased awareness about the importance of influenza vaccination, growing government initiatives for influenza vaccination, and the rising number of clinical trials focused on influenza vaccines.
Based on routes of administration, the market is segmented into intramuscular (IM), subcutaneous (SC), oral, and other routes of administration. In 2024, the intramuscular (IM) segment is expected to account for the largest share of 89.5% of the viral vaccines market. Intramuscular injections are administered into muscle tissue below the dermis and subcutaneous tissue. Intramuscular injections are administered into muscle tissue beneath the dermis and subcutaneous layers and are typically given at two routinely recommended sites. The large market share of this segment is attributed to factors such as its ability to produce the desired immune response, its safety and tolerability, and its use for most inactivated vaccines. In May 2023, Merck & Co., Inc. (U.S.) received approval from the U.S. Food and Drug Administration (FDA) for the intramuscular administration of its MMRV vaccine family, including M-M-RII (Measles, Mumps, and Rubella Virus Vaccine Live), VARIVAX (Varicella Virus Vaccine Live), and ProQuad (Measles, Mumps, Rubella, and Varicella Virus Vaccine Live).
Based on packaging, the market is segmented into vials and prefilled syringes. In 2024, the vials segment is expected to account for a larger share of 93.2% of the viral vaccines market. Vials are small containers, typically made of plastic or glass, used to store liquid medications or powders. The large market share of this segment is driven by factors such as the protective environment vials provide, which helps maintain the stability and efficacy of vaccines by shielding them from moisture, light, and contamination. Vials also allow for visual inspection of contents before administration and feature tamper-proof packaging to prevent unauthorized access or tampering.
In 2024, North America is expected to account for the largest share of 45.6% of the viral vaccines market, followed by Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America’s large market share is attributed to several factors, including the presence of leading manufacturers, significant investments in R&D, strong government and regulatory support, a rising number of infections, the availability of healthcare professionals to administer vaccines, and expanding immunization programs. For instance, in March 2021, Sanofi S.A. (Italy) announced a USD 925 million investment to expand its vaccine manufacturing operations in Toronto, Canada. This investment aims to boost the supply of Sanofi’s FLUZONE High-Dose Quadrivalent influenza vaccine, enhancing its antigen and filling capacity. The new facility will also serve as an international production and distribution hub for Sanofi's high-dose seasonal influenza vaccine.
However, Asia-Pacific is slated to register the highest CAGR of 10. 2% during the forecast period. This growth is driven by factors such as the rising emergence of infectious diseases, a large patient population, increasing public awareness of vaccination, and strong governmental support for immunization. For example, in October 2023, Singapore’s Health Sciences Authority (HSA) approved the updated Spikevax vaccine from Moderna, Inc. (U.S.) for COVID-19 prevention in individuals aged 6 months and older. Additionally, in March 2022, the Government of Thailand, in collaboration with the Pediatric Infectious Disease Society of Thailand (PIDST), launched an expanded immunization program (EPI) that now includes vaccines for BCG, HB, DTwP, Hib, OPV, Rota, MMR, Live JE, Influenza, and HPV for children.
The report offers a competitive analysis based on an extensive assessment of the leading players’ product portfolios and geographic presence and the key growth strategies adopted by them over the past 3–4 years. Some of the key players operating in the viral vaccines market are Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), AstraZeneca plc (U.K.), CSL Limited (Australia), Merck & Co., Inc. (U.S.), Sanofi (France), Sinovac Biotech Ltd. (China), Moderna, Inc. (U.S.), Valneva SE (France), Dynavax Technologies Corporation (U.S.), EMERGENT BIOSOLUTIONS INC. (U.S.), Johnson & Johnson (U.S.), Bharat Biotech Ltd. (India), and Serum Institute of India Pvt. Ltd. (India).
|
Particulars |
Details |
|
Number of Pages |
239 |
|
Format |
|
|
Forecast Period |
2024–2031 |
|
Base Year |
2023 |
|
CAGR (Value) |
9.3% |
|
Market Size (Value) |
USD 64.18 Billion by 2031 |
|
Segments Covered |
By Form
By Vaccine Type
By Route of Administration
By Approach
By Indication
By Packaging
|
|
Countries Covered |
North America (U.S., Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, Ireland, Denmark, Belgium, Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Rest of Asia-Pacific), Latin America (Brazil, Mexico, Rest of Latin America), and Middle East & Africa |
|
Key Companies |
Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), AstraZeneca plc (U.K.), CSL Limited (Australia), Merck & Co., Inc. (U.S.), Sanofi (France), Sinovac Biotech Ltd. (China), Moderna, Inc. (U.S.), Valneva SE (France), Dynavax Technologies Corporation (U.S.), EMERGENT BIOSOLUTIONS INC. (U.S.), Johnson & Johnson (U.S.), Bharat Biotech Ltd. (India), and Serum Institute of India Pvt. Ltd. (India) |
This market study covers the market sizes & forecasts of the viral vaccines market based on form, vaccine type, route of administration, indication, application, packaging, and geography. This market study also provides the value analysis of various segments and subsegments of the global viral vaccines market at the regional & country levels.
The viral vaccines market is projected to reach $64.18 billion by 2031, at a CAGR of 9.3% during the forecast period.
The liquid vaccines segment is expected to account for the largest share of the market in 2024. The large market share of this segment is attributed to factors such as reduced risk of errors, reduced wastage, improved patient experience, low production costs, and better stability.
Based on approach, the therapeutic vaccines segment is slated to register the highest CAGR in the market. The growth of the segment is attributed to factors such as the introduction of antiviral immunity to alter the course of the disease, high specificity and safety, and low costs of vaccines.
The growth of the viral vaccines market is driven by the increasing government focus on immunization programs, technological advances in vaccine administration, and the use of inactivated vaccines in surgeries and treatments.
Furthermore, the growing focus on therapeutic vaccines, this increasing use of adjuvants in vaccines, growth prospects in emerging markets, and the development of highly stable and energy-efficient ultra-low-temperature freezers are expected to generate growth opportunities for market stakeholders.
The key players profiled in the viral vaccines market report are Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), AstraZeneca plc (U.K.), CSL Limited (Australia), Merck & Co., Inc. (U.S.), Sanofi (France), Sinovac Biotech Ltd. (China), Moderna, Inc. (U.S.), Valneva SE (France), Dynavax Technologies Corporation (U.S.), EMERGENT BIOSOLUTIONS INC. (U.S.), Johnson & Johnson (U.S.), Bharat Biotech Ltd. (India), and Serum Institute of India Pvt. Ltd. (India).
Countries like China, Brazil, and India are expected to offer significant growth opportunities for the vendors in this market during the analysis period. This is due to factors such as the rising number of pharmaceutical and biopharmaceutical companies, growing patient population, increasing disposable income, rising awareness of vaccinations, growing immunization programs, and the increasing incidence of infectious diseases.
Viral Vaccines Market is Expected to Grow at a CAGR of 9.3% from 2024 to 2031 to Reach $64.18 Billion by 2031, according to Meticulous Research®.
According to this latest publication from Meticulous Research®, the global viral vaccines market is expected to grow at a CAGR of 9.3% from 2024 to 2031 to reach $64.18 billion by 2031. The growth of this market is driven by the increasing government focus on immunization programs, technological advances in vaccine administration, and the use of inactivated vaccines in surgeries and treatments. However, the high costs of vaccine development and the long timelines of vaccine manufacturing restrain the growth of this market.
Furthermore, the growing focus on therapeutic vaccines, this increasing use of adjuvants in vaccines, growth prospects in emerging markets, and the development of highly stable and energy-efficient ultra-low-temperature freezers are expected to generate growth opportunities for market stakeholders. However, product recalls and inadequate access to vaccines are major challenges impacting the market’s growth.
Pfizer, Inc. (U.S.)
Founded in 1942 and headquartered in New York, U.S., Pfizer Inc. is a leading biopharmaceutical company engaged in the discovery, development, manufacturing, marketing, sale, and distribution of biopharmaceutical products. The company operates primarily in two segments: Biopharma and Business Innovation. Within the Biopharma segment, Pfizer has structured its operations into three key divisions: the Pfizer Oncology Division, the Pfizer U.S. Commercial Division, and the Pfizer International Commercial Division. The company offerings are categorized into Primary Care, Specialty Care, and Oncology. Notably, Pfizer's Primary Care division plays an active role in the viral vaccines market.
Pfizer has a strong geographic presence, with operations in China, Japan, Ireland, Belgium, Germany, Singapore, India, and other countries. The company operates 37 manufacturing plants across the globe, with production facilities in countries such as Belgium, Germany, India, Ireland, Italy, Japan, Singapore, and the U.S., along with multiple distribution centers worldwide. Key subsidiaries include Agouron Pharmaceuticals, LLC (U.S.), Pfizer Pharmaceuticals Israel Ltd. (Israel), Pfizer S.r.l. (Italy), Pfizer PFE Australia Pty Ltd (Australia), Arena Pharmaceuticals Development GmbH (Switzerland), Pfizer Japan Inc. (Japan), Pfizer Limited (U.K.), Pfizer Laboratories Limited (Kenya), and Pfizer (China) Research and Development Co. Ltd. (China).
GlaxoSmithKline plc (U.K.)
Founded in 2000 and headquartered in Brentford, U.K., GlaxoSmithKline (GSK) plc is a biopharmaceutical company engaged in the research, development, identification, and testing of discoveries, as well as the manufacturing and commercialization of medicines. The company operates across three main business segments: Vaccines, Specialty Medicines, and General Medicines. Through its Vaccines segment, GSK offers a variety of viral vaccines. The company employs a broad range of vaccine technologies, including MAPS, mRNA, and adjuvants, to develop tailored vaccines for various diseases.
GSK plc has a geographic presence across 75 countries worldwide, such as the U.A.E, Kenya, Argentina, Brazil, Canada, Mexico, Israel, Bangladesh, India, China, New Zealand, Singapore, Japan, and Malaysia, among others. The company has 33 manufacturing sites and four global R&D centers. As of December 2023, 22,000 worldwide suppliers are associated with the company for the distribution cycle. Some of the company’s major subsidiaries are Affinivax, Inc. (U.S.), GlaxoSmithKline Pharmaceuticals Ltd (India), GSK Vaccines GmbH (Germany), GSK Vaccines S.r.l. (Italy), GSK Pharma India Private Limited (India), GSK Life Sciences FZE (UAE), and SmithKline Beecham Pharmaceuticals Co. (U.S.).
AstraZeneca plc (U.K.)
Formed in 1999 after the merger of Astra AB and Zeneca PLC and headquartered in Cambridge, U.K., AstraZeneca plc is a pharmaceutical business company involved in the research, development, manufacturing, and commercialization of medicines. The company operates its pharmaceuticals business through a single segment. It focuses on therapy areas like Oncology, Biopharmaceuticals, Cardiovascular, Renal & Metabolism (CVRM), Respiratory & Immunology, and Vaccines & Immune Therapies and Rare Diseases. The company offers viral vaccines through its Vaccines & Immune Therapies segment.
AstraZeneca plc has a geographic presence across 125 countries worldwide. The company has a commercial operational presence in over 85 countries, including U.S., Belgium, Bulgaria, France, Italy, Poland, Sweden, Croatia, Germany, Latvia, Portugal, Switzerland, Australia, Canada, Japan, New Zealand, Brazil, Hong Kong, Mongolia, South Africa, Vietnam, and many others. Major subsidiaries of the company include AstraZeneca Ireland Limited (Ireland), Simesa SpA (Italy), AstraZeneca Pharmaceuticals Limited (Kenya), Alexion Europe SAS (France), Portola Netherlands B.V. (Netherlands), AstraZeneca Korea Co. Ltd (South Korea), AstraZeneca AB (Sweden), Ardea Biosciences, Inc. (U.S.), and AstraZeneca Pharma India Limited (India).
CSL Limited (Australia)
Founded in 1916 and headquartered in Melbourne, Australia, CSL Limited is a biotechnology company with a dynamic portfolio of life-saving medicine to treat hemophilia and immune deficiencies, vaccines to prevent influenza, and therapies in iron deficiency and nephrology. The company focuses on three major areas of health: rare and serious diseases, influenza vaccines, and iron deficiency and nephrology.
The company operates in three businesses: CSL Behring, CSL Seqirus, and CSL Vifor. Through the vaccines of the CSL Seqirus business, the company provides viral vaccines. This business is majorly involved in preventing influenza globally and is a transcontinental partner in pandemic preparedness. The company provides medicines and products to patients in 100 countries and employs more than 32,000 people.
The company has a robust geographical presence worldwide. Some of the subsidiaries of the company are Seqirus (Australia) Pty Ltd (Australia), Seqirus Pty Ltd (Australia), Laboratorios Seqirus S.A. (Argentina), Seqirus Canada Inc (Canada), Seqirus Korea Limited (South Korea), Seqirus Netherlands B.V. (Netherlands), Seqirus GmbH (Germany), Seqirus S.r.l (Italy), Seqirus Spain, S.L. (Spain), Seqirus AG (Switzerland), Seqirus Limited (U.K.), Seqirus Inc. (U.S.), among others.
Merck & Co., Inc. (U.S.)
Founded in 1891 and headquartered in New York, U.S., Merck & Co., Inc. is a global healthcare company focused on delivering innovative health solutions through its prescription medicines, including biologic therapies, vaccines, and animal health products. The company operates globally through two major segments: Pharmaceutical and Animal Health. The Pharmaceutical segment encompasses human health pharmaceuticals and vaccine products, with viral vaccines offered through this division. Merck sells its human health vaccines to physicians, wholesalers, distributors, and government entities.
The company has a geographic presence across 141 countries. The Healthcare business segment has 52 manufacturing sites and over 100 distribution centers. Some of its subsidiaries are Merck Healthcare Germany GmbH (Germany), Chord Therapeutics SA (Switzerland), Merck Healthcare Pty. Ltd. (Australia), Merck Biopharma Co., Ltd. (Japan), Merck Healthcare Vietnam Limited (Vietnam), and Merck Healthcare and Life Science Limited (Kenya).
Moderna, Inc. (U.S.)
Founded in 2010 and headquartered in Massachusetts, U.S., Moderna is a provider of messenger RNA (mRNA) vaccines. The company’s mRNA platform enables the development of therapeutics and vaccines for autoimmune diseases, infectious diseases, immuno-oncology, and rare diseases. The company is focused on multiple disease areas and advancing a broad and diverse product portfolio and therapeutics pipeline. The company’s current pipeline includes 45 therapeutics and vaccines, out of which nine are in late-stage development. The company was the earliest to develop COVID-19 vaccines. The company has 230 solely owned patents in the U.S. and more than 170 granted patents outside the U.S.
The company’s major subsidiaries include Moderna Australia Pty Ltd (Australia), Moderna Biopharma Canada Corporation (Canada), Moderna Biotech Kenya Manufacturing Ltd. (Kenya), Moderna Biotech Manufacturing UK Ltd (U.K.), Moderna Biotech Singapore Pte. Ltd. (Singapore), Moderna Biotech Spain, S.L.U. (Spain), Moderna Denmark ApS (Denmark), Moderna Germany GmbH (Germany), Moderna Italy S.r.l. (Italy), Moderna Japan Co., Ltd. (Japan), Moderna US, Inc. (Delaware), and Moderna Taiwan Co., Ltd (Taiwan).
Johnson & Johnson (U.S.) :
Founded in 1886 and headquartered in New Jersey, U.S., Johnson & Johnson is a healthcare company engaged in the research & development, manufacturing, and sale of healthcare products. The company operates through two business segments, namely Innovative Medicine and MedTech. The Innovative Medicine segment is focused on therapeutic areas such as immunology, infectious diseases, neuroscience, oncology, cardiovascular health, metabolism, and pulmonary hypertension. Johnson & Johnson provides viral vaccines through the Innovative Medicine segment.
Johnson & Johnson has a geographic presence across the U.S., Europe, the Western Hemisphere, Africa, Asia, and the Pacific region. Furthermore, the company has 61 facilities globally, of which 18 facilities are used by the Innovative Medicine segment, and the other 43 facilities are used by the MedTech segment. The company’s major subsidiaries include Actelion Pharmaceuticals US, Inc. (U.S.), Anakuria Therapeutics, Inc. (U.S.), Biosense Webster, Inc. California (U.S.), Janssen Biotech, Inc. (U.S.), Actelion Pharmaceuticals Ltd (Switzerland), Ethicon Women's Health & Urology Sarl (Switzerland), GBSC Division of Janssen Biologics B.V. (Netherlands), and Guangzhou Bioseal Biotech Co., Ltd. (China).
EMERGENT BIOSOLUTIONS INC. (U.S.)
Founded in 1998 and headquartered in Maryland, U.S., EMERGENT BIOSOLUTIONS INC. is a specialty biopharmaceutical & life sciences company focused on protecting and enhancing life by providing products and solutions addressing accidental, deliberate, and naturally occurring Public Health Threats (PHTs). The company operates through 3 reportable business segments, namely Commercial Products, MCM Products, and Services. The company provides viral vaccine products through the MCM Products segments.
The company has a geographical presence across North America in Lansing, Michigan, Maryland, Massachusetts, Winnipeg, and Manitoba through its 17 manufacturing facilities and across Europe, the Middle East, Asia, and the Pacific Rim through its distributors. The company’s major domestic subsidiaries include Emergent Commercial Operations Frederick Inc. (Maryland), Emergent Biodefense Operations Lansing LLC (Delaware), Emergent Europe Inc. (Delaware), Emergent International Inc. (Delaware), Emergent Product Development Gaithersburg Inc. (Delaware), Emergent Protective Products USA Inc. (Delaware), and Emergent Travel Health Inc. (Delaware).
Bharat Biotech Ltd. (India)
Formed in 1996 and headquartered in Hyderabad, India, Bharat Biotech Ltd. is a developer of innovative vaccines and bio-therapeutics. The company owns 220 Patents and has a geographic presence in over 125 countries. The company’s manufacturing facilities have been approved by the U.S. FDA, KFDA, and WHO.
The company’s manufacturing facilities are located in Telangana, Gujarat, Karnataka, and Maharashtra in India. The company’s manufacturing facilities use microbial cultures such as E.coli and recombinant yeast. Apart from microbes, the company also uses raw materials such as peptone, sucrose, EDTA, Tris Buffer, and sodium chloride.
Sinovac Biotech Ltd. (China)
Incorporated in 2001 and headquartered in Beijing, China, Sinovac Biotech Ltd. is a biopharmaceutical company engaged in the research, development, production, and marketing of vaccines that protect against infectious diseases. The company operates through one reportable business segment and serves three business markets, namely, Expanded Program of Immunization (EPI), Private Pay, and Export. The EPI includes vaccines that are inoculated to residents with government provisions. The Private Pay market includes other vaccines that are voluntarily inoculated by individuals.
The company has a strong geographic presence in China and internationally in Singapore, Hong Kong, Thailand, the Philippines, Bangladesh, Indonesia, and Chile. The company has manufacturing sites, R&D centers, and production sites primarily in China. Some of the company’s subsidiaries are Sinovac Beijing Co., Ltd. (China), Sinovac (Dalian) Vaccine Technology Co., Ltd. (China), Sinovac Life Sciences Co., Ltd. (China), and Sinovac Biomed Co., Lt
Acess Full Report @ https://www.meticulousresearch.com/product/viral-vaccines-market-5920
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: May-2024
Published Date: Jan-2021
Published Date: Mar-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates