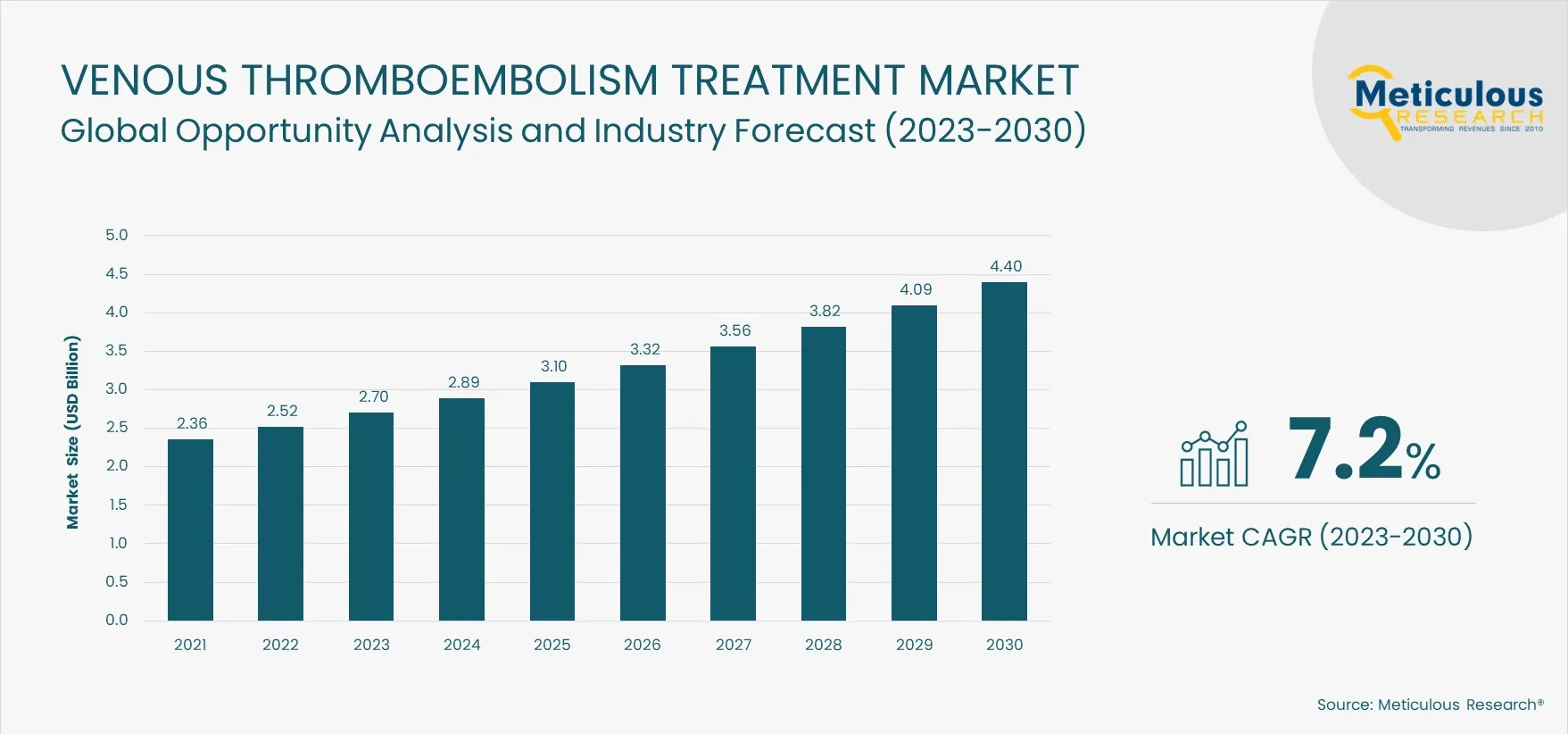
The Venous Thromboembolism Treatment Market is expected to grow at a CAGR of 7.2% from 2023 to 2030 to reach $4.40 billion by 2030. The growth of this market is mainly driven by the high prevalence of venous thromboembolism, rising incidence of cancer, growing prevalence of secondary risk factors such as diabetes and obesity, increase in orthopedic surgical procedures, and the rising demand for minimally invasive treatment procedures. Furthermore, the growing accessibility and affordability of treatment options, advancements in VTE diagnosis and treatment methods, and the market expansion in emerging economies are expected to create market growth opportunities.
However, instances of product failure and product recalls may restrain the market’s growth. Additionally, lack of awareness about venous thromboembolism and increasing awareness of probable side effects of compression garments are major challenges for market growth.
Here are the top 10 companies operating in the Venous Thromboembolism Treatment Market
Boston Scientific Corporation (U.S.)
Founded in 1979 and headquartered in Massachusetts, U.S., Boston Scientific Corporation develops, manufactures, and markets medical devices. These medical devices are used in a broad range of interventional medical specialties to provide medical solutions and improve the health of patients around the world. The company develops minimally invasive medical technologies. It operates through two major segments: MedSurg and Cardiovascular. These two segments are further divided into sub-segments as per the product portfolio. MedSurg is divided into Endoscopy, Urology, and Neuromodulation sub-segments, and the Cardiovascular segment is divided into Cardiology and Peripheral Interventions sub-segments. The company operates in the venous thromboembolism treatment market through the Cardiovascular segment.
The company has a geographic presence in regions such as North America, Europe, Asia-Pacific, and Latin America, specifically in countries like Canada, China, the Netherlands, India, Japan, and the U.S. Some of the subsidiaries of the company are Boston Scientific (UK) Limited (U.K.), Boston Scientific AG (Switzerland), Boston Scientific do Brasil Ltda. (Brazil), Boston Scientific India Private Limited. (India), Boston Scientific International S.A. (France), and Apollo Endosurgery, Inc. (U.S.).
Stryker Corporation (U.S.)
Incorporated in 1941 and headquartered in Michigan, U.S., Stryker Corporation offers products and services in Medical and Surgical, Neurotechnology, Orthopedics, and Spine-related treatments. The company operates through two reportable segments, including MedSurg and Neurotechnology and Orthopaedics and Spine. MedSurg products include surgical equipment, patient and caregiver safety technologies, navigation systems (Instruments), and other products. Neurotechnology includes neurosurgical, neurovascular, and craniomaxillofacial implant products. The company offers venous thromboembolism treatment market through the MedSurg and Neurotechnology segment.
The company owns approximately 4,800 patents in the U.S. and approximately 7,300 patents in other countries. The company has a presence in more than 75 countries globally, including the U.S., Mexico, Italy, Malaysia, South Africa, India, and other countries. Some of the company’s subsidiaries are Berchtold Holding Switzerland GmbH (Switzerland), Cartiva, Inc. (U.S.), and EnMovi, Ltd. (U.S.).
Founded in 1979 and headquartered in Ohio, U.S., Cardinal Health, Inc. is a healthcare services and products company that offers pharmaceuticals and medical products. Cardinal Health, Inc. operates through two reportable segments, namely Pharmaceutical and Medical. The company operates in the venous thromboembolism treatment market through the Medical segment.
The company offers a range of products and solutions catering to diverse healthcare settings, including hospitals, healthcare systems, pharmacies, ambulatory surgery centers, clinical laboratories, and physicians’ offices. Additionally, it extends its solutions to patients at home through its dedicated Health atHome Solutions division.
The company has a distribution network in countries outside the U.S., including Germany, Ireland, the Philippines, Switzerland, the U.K., Brazil, Denmark, France, Japan, Netherlands, Spain, and Sweden, among others. Some of the subsidiaries are Cardinal Health Germany 507 GmbH (Germany), Cardinal Health Ireland 508 Limited (Ireland), Cardinal Health Mexico 514 S. de R.L. de C.V. (Mexico), Cardinal Health Japan G.K. (Japan), Cardinal Health Netherlands 502 B.V. (Netherlands), and Cardinal Health France 506 SAS (France).
Enovis Corporation (U.S.)
Founded in 1978 and headquartered in Texas, U.S., Enovis Corporation develops and manufactures products used for reconstructive surgery, rehabilitation, pain management, and physical therapy. The company operates through two reportable segments: Prevention & Recovery and Reconstructive segments. The Prevention & Recovery segment consists of rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management, and physical therapy products. The company offers venous thromboembolism treatment products through the Prevention & Recovery segment.
The company’s brands include DVT, DonJoy, Saunders, Aircast, Chattanooga, and Enovis Surgical. It markets its products to a diverse range of end users, including orthopedic specialists, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers, and various healthcare professionals. The company has established its presence in key markets such as the U.S., Australia, France, Italy, India, and several other countries.
Founded in 1949 and headquartered in Dublin, Ireland, Medtronic is a healthcare technology company providing services to physicians, clinicians, and patients. The company is engaged in R&D and manufactures and sells biomedical engineering products that help alleviate pain, restore health, and extend life.
The company operates through four reportable segments: Cardiovascular Portfolio, Medical Surgical Portfolio, Neuroscience Portfolio, and Diabetes Operating Unit. The company operates in the venous thromboembolism treatment market through the Cardiovascular Portfolio segment. The company’s products are primarily used in hospitals, surgical centers, and alternate care facilities, such as home care and long-term care facilities. The company markets its products to material managers, group purchasing organizations (GPOs), and integrated delivery networks (IDNs).
The company has a wide geographic presence and offers products in over 150 countries. The company’s four largest markets are the U.S., Western Europe, China, and Japan. Medtronic plc has subsidiaries in Australia, Brazil, the U.S., Canada, China, Egypt, France, Germany, Italy, Mexico, and the Netherlands. Some of the major subsidiaries are Medtronic Australasia Pty Ltd. (Australia), Medtronic BioPharma B.V. (Netherlands), and Medtronic Canada ULC (Canada).
Cook Group Incorporated (U.S.)
Founded in 1963 and headquartered in Indiana, U.S., Cook Group Incorporated designs and manufactures products and technologies that move to eliminate the need for open surgery. The company combines medical devices with biological materials and cellular therapies to deliver better outcomes. The company provides products to over 135 countries and has over 12,000 employees.
Cook Group Incorporated operates through five major businesses, namely Medical Devices, Life Sciences, Services, Property Management, and Resorts. They operate in the venous thromboembolism treatment market through the Medical Devices segment. This segment is further segmented into two divisions: Cook Medical Vascular Division and Cook Medical MedSurg Division.
The Cook Vascular Division is focused on therapies in Aortic Intervention, Interventional Radiology, Lead Management, and Peripheral Intervention. Venous thromboembolism treatment and prevention devices are offered through the Peripheral Intervention division.
It offers products in over 60 clinical specialties, such as Acute Care Surgery, Cardiovascular Surgery, Critical Care Medicine, Electrophysiology, Vascular Access, Vascular Medicine, and Vascular Surgery, among others.
Koninklijke Philips N.V. (Netherlands)
Founded in 1891 and headquartered in Eindhoven, Netherlands, Koninklijke Philips N.V. is involved in developing, manufacturing, and distributing diagnosis and treatment solutions that support precision diagnosis and minimally invasive interventions and therapy, health IT solutions, products for oral healthcare solutions, mother and child-care solutions, and personal care. Philips operates through four reportable business segments: Personal Health, Diagnosis & Treatment, Connected Care, and Other. The Diagnosis & Treatment segment comprises Ultrasound, Diagnostic Imaging, Image Guided Therapy, and Enterprise Diagnostic Informatics. The company operates in the venous thromboembolism treatment market through its Image Guided Therapy segment.
The company also provides training in Venous thromboembolism (VTE), deep vein thrombosis (DVT), and cardiopulmonary embolism disease diagnosis and treatment through Philips Education Services.
Koninklijke Philips N.V. has a geographical presence in more than 100 countries globally, including the U.S., Netherlands, China, Japan, the U.K., France, Germany, and other countries. The company has several manufacturing sites in North America, including New York, California, and Pennsylvania. The company has four regional innovation hubs in the U.S., India, Europe, and China.
AngioDynamics, Inc. (U.S.)
Founded in 1988 and headquartered in New York, U.S., AngioDynamics, Inc. is a medical technology company focused on expanding cancer treatment options through various products and devices. The company operates through two business segments: Med Tech and Med Device. Venous thromboembolism treatment and prevention solutions are offered through both segments.
The company has a direct sales force in the U.S. and a combination of direct sales and distributor relationships for international sales in several countries, including the U.K., Netherlands, France, Canada, Brazil, and Israel.
Some of the major subsidiaries include AngioDynamics UK Limited (U.K.), AngioDynamics Netherlands B.V. (Netherlands), RITA Medical Systems, LLC (Delaware), AngioDynamics France, SARL (France), AngioDynamics Canada, Inc. (Canada), AngioDynamics Medical Brasil Servicos de Marketing Ltda. (Brazil), RadiaDyne LLC (Texas), Eximo Medical Ltd. (Israel), and AngioDynamics VA LLC (Delaware).
Founded in 1957 and headquartered in Malmö, Sweden, ArjoHuntleigh AB manufactures medical devices and offers solutions to improve the quality of life for people with reduced mobility and age-related health challenges. The company operates through two segments: Global Sales and North America.
The company has five product development and production units in Canada, the Dominican Republic, China, Poland, and the U.K. and provides sales and service operations in more than 100 countries.
The company was acquired by Getinge AB (Sweden) in 1995 and was considered the Extended Care business area of Getinge. For several years, Arjo represented the Extended Care business area. However, in 2016, Getinge AB announced the division of the company into two operations: Getinge and Arjo.
The company has several offices globally in countries including Australia, Belgium, Brazil, Denmark, Dominican Republic, France, U.A.E., Hong Kong, India, Ireland, Italy, Japan, Canada, China, Mexico, Netherlands, New Zealand, and Poland.
LifeTech Scientific Corporation (China)
Founded in 1999 and headquartered in Shenzhen, China., LifeTech Scientific Corporation develops, manufactures, and markets advanced minimally invasive interventional medical devices for cardiovascular and peripheral vascular diseases and disorders. The company’s offerings are categorized as structural heart diseases business, peripheral vascular diseases business, cardiac pacing, and electrophysiology business.
Peripheral vascular disease-related products mainly include vena cava filters and stent-grafts. The company offers venous thromboembolism treatment solutions through the peripheral vascular diseases business.
The company has subsidiaries and sales offices in six countries, and its global sales network covers more than 100 countries and regions. Some of the company’s subsidiaries are Lifetech Scientific International Holding Limited (China), Lifetech Shenzhen (China), and Biotyx Medical (Shenzhen) Co., Ltd. (China).