Resources
About Us
South East Asia Pharmaceutical Contract Development and Manufacturing Market by Service {Manufacturing [API, FDF (Parenteral, Tablet, Capsule, Oral Liquid)], Drug Development, Biologics Manufacturing, Packaging}, End User [Large Pharma] - Forecast to 2030
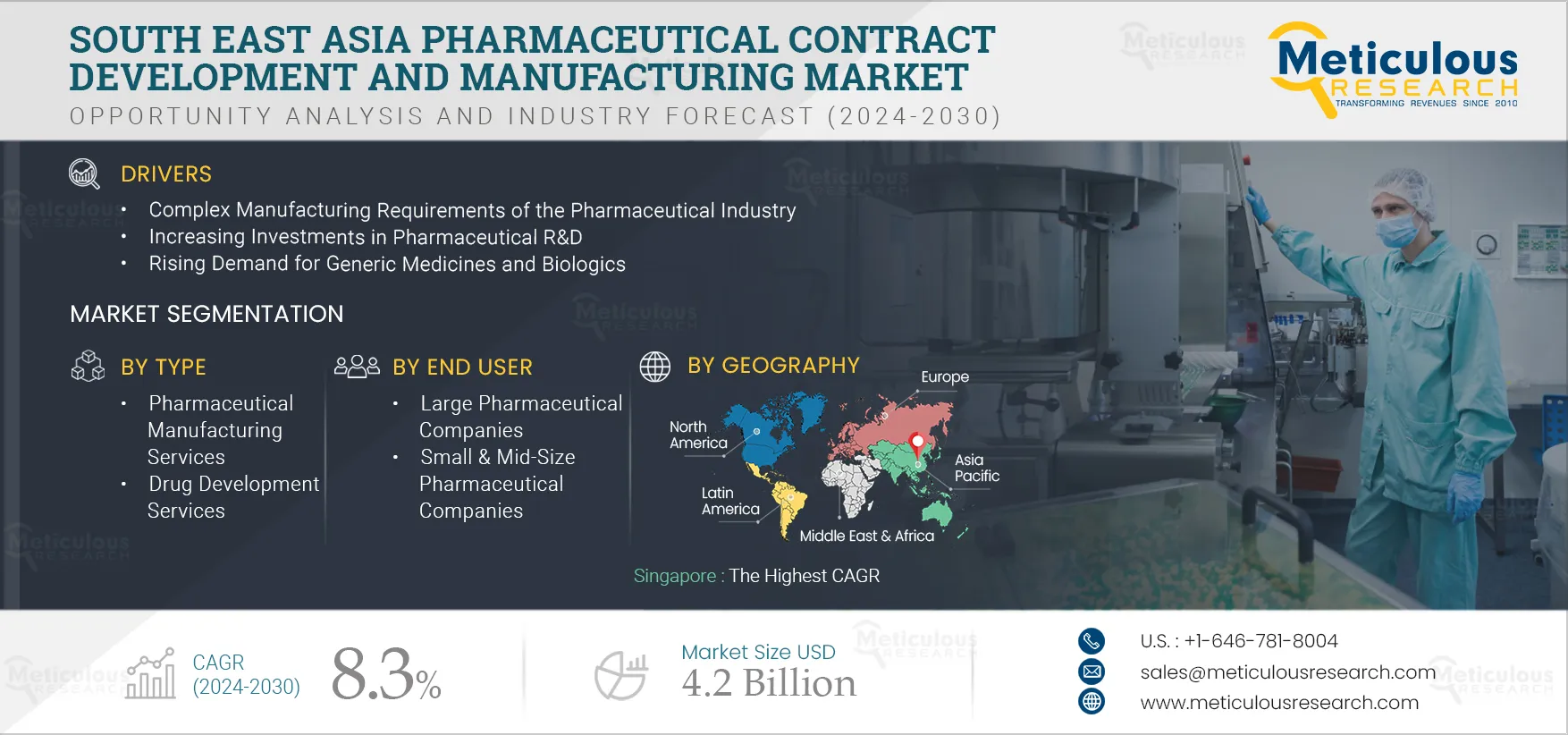
Report ID: MRHC - 104992 Pages: 150 Jul-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe South East Asia Pharmaceutical Contract Development and Manufacturing Market is expected to reach $4.2 billion by 2030 at a CAGR of 8.3% from 2024 to 2030. Pharmaceutical contract development and manufacturing organizations offer various services based on a contract to small or large pharmaceutical companies. These services include active pharmaceutical ingredient (API) manufacturing, finished dosage form (FDF) manufacturing, drug development & drug discovery services, and biologics manufacturing services. The COVID-19 pandemic positively impacts the pharmaceutical contract development and manufacturing market due to the increased demand for pharmaceutical products such as tablets, capsules, drugs, and vaccines.
The growth of the South East Asia pharmaceutical contract development and manufacturing market is attributed to the complex manufacturing requirements of the pharmaceutical industry, increasing investment in pharmaceutical R&D, rising demand for generic medicines and biologics, patent expiration, and increasing government funding for drug development and research. In addition, the growing demand for cell and gene therapies and personalized medicines and growth in high potency active pharmaceutical ingredients (HPAPI) and antibody-drug conjugates (ADC) markets are expected to offer significant opportunities for the growth of the pharmaceutical contract development and manufacturing market. However, the lack of skilled professionals is challenging the growth of this market.
Healthcare R&D activities have significantly increased in the last two to three decades with rising investments from various government organizations and pharmaceutical companies. This funding is mainly driven by the rising prevalence of chronic diseases due to the growing geriatric population, complexities in clinical trials, and higher failure of drugs in early-phase studies. Government of South East Asia countries are increasing their funding for boosting pharmaceutical and biopharmaceutical research. In addition, several initiatives have been taken by the governments of respective countries to boost pharmaceutical R&D. For instance, the Singapore government, in 2018, introduced the Pharma Innovation Programme Singapore (PIPS). The program aims to bring the pharmaceutical industry and public research sector capabilities to increase the operational efficiency in Singapore’s pharmaceutical industry. The program has commenced operations in 2021.
Furthermore, pharmaceutical companies have also increased their spending on R&D. The extent of pharmaceutical R&D spending is an important metric to show a company's commitment to finding new drugs. Therefore, increasing government investments in pharmaceutical R&D is expected to expand the research pipeline, enhancing the number of products converted to usable forms with the help of pharmaceutical contract manufacturers and driving market growth. For instance, in 2021, Lonza Group AG (Switzerland) invested in expanding mammalian development services in Singapore. This investment will expand the current laboratory capabilities by establishing a new 1,800 Sq meter laboratory at Singapore Science Park.
Click here to: Get Free Sample Pages of this Report
The increasing prevalence of cancer, autoimmune, cardiovascular, and infectious diseases in South East Asia countries has resulted in the emergence of biopharmaceuticals or biologics as an important therapeutic class for treating these diseases. For instance, in 2022, the total cases registered for dengue in Vietnam were 361,813, with 133 deaths (Source: Vietnam Ministry of Health). The cases increased five-fold compared to 2021. Biologics offer numerous advantages over synthetic drugs by targeting specific sites and rarely causing side effects.
According to the International Diabetes Federation (IDF), as of 2021, Indonesia has the fifth-highest number of people with diabetes worldwide. This results in increasing approvals and the demand for biologics, such as therapeutic proteins and monoclonal antibodies (mAbs).
Furthermore, the COVID-19 pandemic and the rising incidences of infectious diseases significantly burdens the healthcare sector and boosts the demand for vaccines. For instance, in 2020, the outbreak of the COVID-19 pandemic imposed a huge burden on the pharmaceutical industry and increased the demand for vaccines and breakthrough drugs. Due to this increase, the demand for biopharmaceutical products has risen with the subsequent growth in the South East Asia pharmaceutical contract development and manufacturing market.
Key Findings of the Market Study:
The Biologics Manufacturing Services Segment is Expected to Grow at Highest CAGR
Based on type, the South East Asia pharmaceutical contract development and manufacturing market is segmented into pharmaceutical manufacturing services, drug development services, and biologics manufacturing services. The biologics manufacturing services segment is expected to grow at the highest CAGR during the forecast period. The growth of the biologics manufacturing services segment is driven by the growing inclination of biopharma and pharma companies towards outsourcing their activities to accelerate drug development workflow and the growth in the adoption of advanced technologies for biologics production. The increasing number of developments in the sector has resulted in pharmaceutical and biopharmaceutical companies outsourcing their manufacturing function to CDMOs.
Additionally, the increasing prevalence of chronic diseases, the increasing advantages of biologics over synthetic drugs, and the demand and approvals for biologics support market growth. Biologics are also majorly used for diabetes management. Victoza and Trulicity are majorly used biologics for diabetes treatment. In 2021, 19.5 million people were living with diabetes in Indonesia, which is expected to reach 28.6 million by 2045 (Source: IDF).
In 2024, the Large Pharmaceutical Companies Segment is Expected to Account for the Largest Share of the Market
Based on end user, the South East Asia pharmaceutical contract development and manufacturing market is segmented into large pharmaceutical companies, small & mid-size pharmaceutical companies, and generic pharmaceutical companies. In 2024, the large pharmaceutical companies segment is expected to account for the largest share of the market. The factors attributing to the largest share of the market are the growing need for state-of-the-art processes and production technologies, the need for business expansion globally, the growing strategic focus of sponsors to focus on core business functions such as marketing and R&D, and continued efforts of pharmaceutical companies to reduce production costs.
Furthermore, the outbreak of the COVID-19 pandemic has led CDMOs to enter into strategic partnerships or agreements for large-scale vaccine development and increase the manufacturing capacity of companies for drugs. In July 2022, WuXi Biologics (China) announced a ten-year plan with an investment of USD 1.4 billion to expand the company’s research, development, and large-scale drug substance and drug product (DS/DP) manufacturing capacity and capabilities in Singapore.
Singapore: Largest Market
Singapore is expected to grow at the highest CAGR during the forecast period in South East Asia pharmaceutical contract development and manufacturing market. Growing government investments in the life sciences industry and the increasing prevalence of chronic diseases are the major factors generating high demand for pharmaceuticals in the country. Additionally, the strategic initiatives undertaken by key players and government agencies are supporting the market growth. In December 2022, GSK plc (U.K.), Takeda Pharmaceutical Company (Japan), and Sanofi S.A. (France) announced a partnership with Singapore’s Agency for Science, Technology, and Research (A*STAR) and various Singapore universities through Biologics Pharma Innovation Programme Singapore (BioPIPS), to enhance the Singapore's biologics manufacturing capabilities, including recombinant therapeutic proteins and vaccines.
Singapore’s infrastructure and Intellectual property protection (IPP) make the territory a suitable offshore location for pharmaceutical and biotechnology companies. Also, the country is well recognized for the quality of its API and bulk drugs, and many companies from mature markets are inclined to use contract manufacturing in Singapore.
Key Players
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments adopted by leading market players in the industry over the years (2020–2024). The key players operating in the South East Asia pharmaceutical contract development and manufacturing market are Aenova Group (Germany), Wuxi Biologics, Inc. (China), Vetter Pharma International GmbH (Germany), Catalent, Inc. (U.S.), Lonza Group Ltd. (Switzerland), Almac Group (U.K.), Eurofins Scientific (France), Thermo Fisher Scientific, Inc. (U.S.), Jubilant Life Science Limited (India), Aurobindo Pharma Ltd. (India), APD Pharmaceutical (Singapore), and Esco Aster Pte. Ltd. (Singapore).
Scope of the Report:
South East Asia Pharmaceutical Contract Development and Manufacturing Market Assessment, by Type
South East Asia Pharmaceutical Contract Development and Manufacturing Market Assessment, by End User
South East Asia Pharmaceutical Contract Development and Manufacturing Market Assessment, by Country
Key questions answered in the report:
The South East Asia pharmaceutical contract development and manufacturing market studies different types of services, such as pharmaceutical manufacturing services, drug development services, and biologics manufacturing services used by end users. The Southeast Asia pharmaceutical contract development and manufacturing market studied in this report involves the analysis of various segments at country levels.
The South East Asia pharmaceutical contract development and manufacturing market is projected to reach $4.2 billion by 2030 at a CAGR of 8.3% from 2024 to 2030.
Among all the types studied in the report, the pharmaceutical manufacturing services segment is expected to account for the largest share of the market in 2024. The large share of the segment is attributed to the growing need to reduce manufacturing costs, bring a new drug to the market quickly at the lowest possible cost, the requirement for high-quality bulk manufacturing, and the growing demand for generic drugs.
The growth of the South East Asia pharmaceutical contract development and manufacturing market is attributed to the complex manufacturing requirements of the pharmaceutical industry, increasing investment in pharmaceutical R&D, rising demand for generic medicines and biologics, patent expiration, and increasing government funding for drug development and research. In addition, the growing demand for cell and gene therapies and personalized medicines and growth in high potency active pharmaceutical ingredients (HPAPI) and antibody-drug conjugates (ADC) markets are expected to offer significant opportunities for the growth of the pharmaceutical contract development and manufacturing market.
The key players operating in the South East Asia pharmaceutical contract development and manufacturing market are Aenova Group (Germany), Wuxi Biologics, Inc. (China), Vetter Pharma International GmbH (Germany), Catalent, Inc. (U.S.), Lonza Group Ltd. (Switzerland), Almac Group (U.K.), Scientific (France), Thermo Fisher Scientific, Inc. (U.S.), Jubilant Life Science Limited (India), Aurobindo Pharma Ltd. (India), APD Pharmaceutical (Singapore), and Esco Aster Pte. Ltd. (Singapore).
Singapore is expected to offer significant growth opportunities owing to the government initiatives to create favorable pharmaceutical drug approval programs, increase investment from existing pharmaceutical manufacturers in the country for infrastructure development, and growing investments towards biosimilar manufacturing.
1. Introduction
1.1. Market Definition & Scope
1.2. Market Ecosystem
1.3. Currency & Limitations
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Approach
2.2. Process of Data Collection and Validation
2.2.1. Secondary Research
2.2.2. Primary Research/Interviews with Key Opinion Leaders of the Industry
2.3. Market Sizing and Forecast
2.3.1. Market Size Estimation Approach
2.3.2. Growth Forecast Approach
2.3.3. Assumptions for the Study
3. Executive Summary
4. Market Insights
4.1. Overview
4.2. Factors Affecting Market Growth
4.2.1. Drivers
4.2.1.1. Complex Manufacturing Requirements of the Pharmaceutical Industry
4.2.1.2. Increasing Investments in Pharmaceutical R&D
4.2.1.3. Rising Demand for Generic Medicines and Biologics
4.2.1.4. Patent Expiration
4.2.1.5. Increasing Government Funding for Drug Development and Research
4.2.2. Restraints
4.2.2.1. Stringent Regulatory Requirements
4.2.3. Opportunities
4.2.3.1. Growing Demand for Cell Therapies, Gene Therapies, and Personalized Medicines
4.2.3.2. Growth in High Potency Active Pharmaceutical Ingredients (HPAPI) and Antibody-drug Conjugates (ADC) Markets
4.2.4. Challenge
4.2.4.1. Shortage of Skilled Professionals
4.2.5. Factor Analysis
4.3. Trends
4.4. Regulatory Analysis
4.5. Porter’s 5 Force Analysis
5. South East Asia Pharmaceutical Contract Development and Manufacturing Market Assessment, by Type
5.1. Overview
5.2. Pharmaceutical Manufacturing Services
5.2.1. Active Pharmaceutical Ingredient (API) Manufacturing Services
5.2.2. Finished Dosage Forms (FDF) Manufacturing Services
5.2.2.1. Parenteral/Injectable Manufacturing Services
5.2.2.2. Tablet Manufacturing Services
5.2.2.3. Capsule Manufacturing Services
5.2.2.4. Oral Liquid Manufacturing Services
5.2.2.5. Other Formulations
5.3. Drug Development Services
5.4. Biologics Manufacturing Services
5.4.1. Active Pharmaceutical Manufacturing Services (API) Manufacturing Services
5.4.2. Finished Dosage Form Manufacturing (FDF) Services
6. South East Asia Pharmaceutical Contract Development and Manufacturing Market Assessment, by End User
6.1. Overview
6.2. Large Pharmaceutical Companies
6.3. Small & Mid-Size Pharmaceutical Companies
6.4. Generic Pharmaceutical Companies
7. South East Asia Pharmaceutical Contract Development and Manufacturing Market Assessment, by Geography
7.1. Overview
7.2. Indonesia
7.3. Vietnam
7.4. Thailand
7.5. Singapore
7.6. Philippines
7.7. Malaysia
7.8. Rest of Southeast Asia
8. Competition Analysis
8.1. Overview
8.2. Key Growth Strategies
8.3. Competitive Benchmarking
8.4. Competitive Dashboard
8.4.1. Industry Leaders
8.4.2. Market Differentiators
8.4.3. Vanguards
8.4.4. Emerging Companies
8.5. Market Share Analysis/Market Ranking, 2022
9. Company Profiles (Company Overview, Financial Overview, Product Portfolio, and Strategic Developments)
9.1. Aenova Group
9.2. Wuxi Biologics, Inc.
9.3. Vetter Pharma International GmbH
9.4. Catalent, Inc
9.5. Lonza Group Ltd.
9.6. Almac Group
9.7. Eurofins Scientific
9.8. Thermo Fisher Scientific, Inc.
9.9. Jubilant Pharmova Limited
9.10. Aurobindo Pharma Ltd
9.11. APD Pharmaceutical
9.12. Esco Aster Pte. Ltd.
(Note: SWOT Analysis of the Top 5 Companies Will Be Provided)
10. Appendix
10.1. Available Customization
10.2. Related Reports
List of Tables
Table 1 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021-2030 (USD Million)
Table 2 Southeast Asia Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 3 Southeast Asia Pharmaceutical Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 4 Southeast Asia Active Pharmaceutical Ingredient (API) Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 5 Southeast Asia Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 6 Southeast Asia Finished Dosage Forms (FDF) Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 7 Southeast Asia Parenteral/Injectable Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 8 Southeast Asia Tablet Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 9 Southeast Asia Capsule Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 10 Southeast Asia Oral Liquid Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 11 Southeast Asia Other Formulations Market, by Country/Region, 2021–2030 (USD Million)
Table 12 Southeast Asia Drug Development Services Market, by Country/Region, 2021–2030 (USD Million)
Table 13 Southeast Asia Biologics Manufacturing Services, by Type, 2021–2030 (USD Million)
Table 14 Southeast Asia Biologics Manufacturing Services, by Country/Region, 2021–2030 (USD Million)
Table 15 Southeast Asia Active Pharmaceutical Manufacturing Services (API) Manufacturing Services Market, by Country/Region, 2021–2030 (USD Million)
Table 16 Southeast Asia Finished Dosage Form Manufacturing (FDF) Services Market, by Country/Region, 2021–2030 (USD Million)
Table 17 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 18 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market for Large Pharmaceutical Companies, by Country/Region, 2021–2030 (USD Million)
Table 19 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market for Small & Mid-Size Pharmaceutical Companies, by Country/Region, 2021–2030 (USD Million)
Table 20 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market for Generic Pharmaceutical Companies, by Country/Region, 2021–2030 (USD Million)
Table 21 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by Country/Region., 2021-2030 (USD Million)
Table 22 Southeast Asia: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 23 Southeast Asia: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 24 Southeast Asia: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 25 Southeast Asia: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 26 Southeast Asia: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 27 Indonesia: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 28 Indonesia: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 29 Indonesia: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 30 Indonesia: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 31 Indonesia: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 32 Vietnam: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 33 Vietnam: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 34 Vietnam: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 35 Vietnam: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 36 Vietnam: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 37 Thailand: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 38 Thailand: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 39 Thailand: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 40 Thailand: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 41 Thailand: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 42 Singapore: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 43 Singapore: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 44 Singapore: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 45 Singapore: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 46 Singapore: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 47 Philippines: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 48 Philippines: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 49 Philippines: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 50 Philippines: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 51 Philippines: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 52 Malaysia: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 53 Malaysia: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 54 Malaysia: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 55 Malaysia: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 56 Malaysia: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 57 Rest of Southeast Asia: Pharmaceutical Contract Development and Manufacturing Market, by Type, 2021–2030 (USD Million)
Table 58 Rest of Southeast Asia: Pharmaceutical Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 59 Rest of Southeast Asia: Finished Dosage Forms (FDF) Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 60 Rest of Southeast Asia: Biologics Manufacturing Services Market, by Type, 2021–2030 (USD Million)
Table 61 Rest of Southeast Asia: Pharmaceutical Contract Development and Manufacturing Market, by End User, 2021–2030 (USD Million)
Table 62 Recent Developments, by Company, 2020—2024
List of Figures
Figure 1 Research Process
Figure 2 Key Secondary Sources
Figure 3 Primary Research Techniques
Figure 4 Key Executives Interviewed
Figure 5 Breakdown of Primary Interviews (Supply Side & Demand Side)
Figure 6 Market Size Estimation
Figure 7 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by Type, 2024 VS. 2030 (USD Million)
Figure 8 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by End User, 2024 VS. 2030 (USD Million)
Figure 9 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by Country, 2024 VS. 2030 (USD Million)
Figure 10 Factors Affecting Market Growth
Figure 11 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by Type, 2024 VS. 2030 (USD Million)
Figure 12 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by End User, 2024 VS. 2030 (USD Million)
Figure 13 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market, by Country, 2024 VS. 2030 (USD Million)
Figure 14 Southeast Asia: Pharmaceutical Contract Development and Manufacturing Market Snapshot
Figure 15 Key Growth Strategies Adopted by Leading Players, 2020—2024
Figure 16 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market: Competitive Benchmarking, by Type
Figure 17 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market: Competitive Dashboard
Figure 18 Southeast Asia Pharmaceutical Contract Development and Manufacturing Market: Market Share Analysis (2022)
Figure 19 Aenova Group.: Financial Snapshot (2022)
Figure 20 Wuxi Biologics, Inc.: Financial Snapshot (2022)
Figure 21 Catalent, Inc.: Financial Snapshot (2022)
Figure 22 Lonza Group Ltd.: Financial Snapshot (2022)
Figure 23 Eurofins Scientific.: Financial Snapshot (2022)
Figure 24 Thermo Fisher Scientific, Inc.: Financial Snapshot (2022)
Figure 25 Jubilant Life Science Limited: Financial Snapshot (2022)
Figure 26 Aurobindo Pharma Ltd.: Financial Snapshot (2022)

























Published Date: Jul-2024
Published Date: Jul-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates