Resources
About Us
South East Asia Molecular Diagnostics Market by Offering (Reagents, Kits, Systems, Software), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (HIV, Influenza, HPV, Oncology, Gene Testing), End User - Forecast to 2031
Report ID: MRHC - 1041092 Pages: 181 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportMolecular diagnostics help detect the targeted genetic material in human, viral, and bacterial genomes. Molecular diagnostic tests are increasingly being used in many areas, including infectious diseases, oncology, and clinical genetics. Advancements in molecular diagnostics will continue to improve the accuracy and speed of diagnosis and will become an essential aspect of patient-tailored interventions and therapeutics.
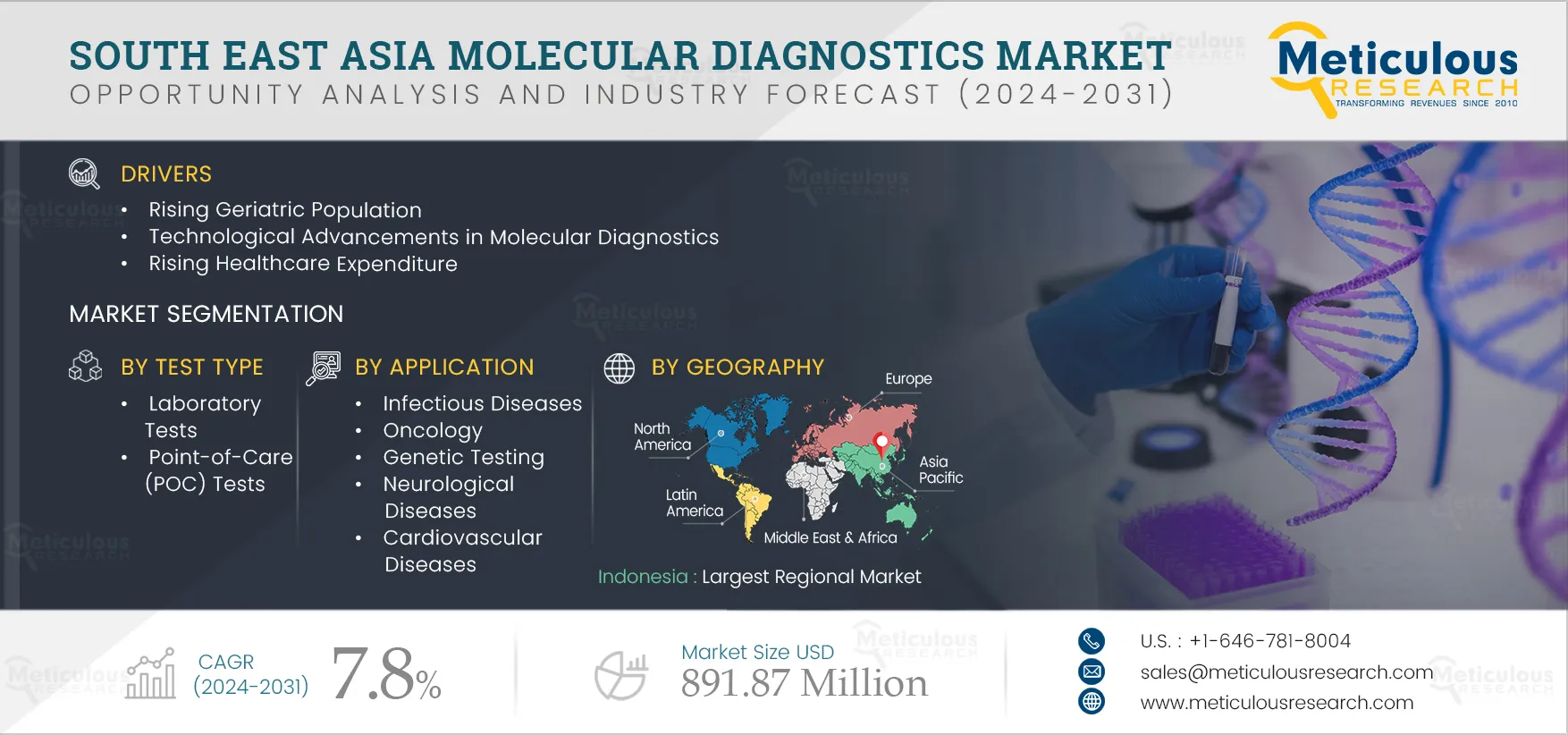
The growth of the South East Asia molecular diagnostics market is driven by several factors, including the rising geriatric population, increasing prevalence of communicable & non-communicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditure. Factors such as emerging medical tourism in South East Asian countries and increasing focus on companion diagnostics are a few of the opportunities that would help grow the market in the future. A shortage of skilled professionals could be considered a challenge for the South East Asia molecular diagnostics market. Moreover, the lack of harmonization of the medical device regulations across South East Asian countries and the high costs of molecular diagnostics tests restrain the growth of the market.
Click here to: Get Free Sample Pages of this Report
The world is becoming increasingly connected, and diseases are spreading rapidly due to cross-border travel. Also, people’s changing lifestyles are impacting their health, increasing the prevalence of non-communicable diseases. For instance, according to the World Health Organization (WHO), in 2020, in South East Asia, an estimated 4.3 million people fell ill with tuberculosis (TB), and around 700,00 million people died from TB. The burden of drug-resistant TB (DR-TB) also increased by 3% between 2020 and 2021, with 450,000 new cases of rifampicin-resistant TB (RR-TB) recorded in 2021.
Similarly, as per GLOBOCAN 2020, countries in South East Asia, such as Indonesia, Thailand, Malaysia, and Vietnam, are expected to witness a rise in cancer cases in the coming years. For instance, in 2020, the number of cancer cases in Indonesia was 396,314, which is expected to rise to 645,436 in 2040. Diagnostic tests are crucial in the medical care of patients suffering from diseases. Thus, the increasing prevalence of communicable and non-communicable diseases is driving the market.
Healthcare expenditure includes all expenditures that allow healthcare systems to function optimally and provide quality care to patients. Increasing healthcare expenditure can enable regular health interventions such as annual medical checkups and preventive screening, which are likely to improve the overall health status of a country. For instance, as per data from the World Bank, in 2020, the health expenditure per capita in Indonesia was USD 132.6, the expenditure per capita in Malaysia was USD 418.66, the expenditure per capita in the Philippines was USD 164.74, the expenditure per capita in Vietnam was USD 166.23, and the expenditure in Thailand was USD 305.09. According to the World Health Organization (WHO), in 2023, the average total healthcare expenditure per capita is USD 544 in ASEAN (Association of South East Asian Nations) countries.
The countries’ increasing healthcare expenditure indicates their growing focus on ensuring that people are healthy and disease-free, which is expected to result in the increased adoption of advanced medical technologies, funding and initiatives for disease diagnosis and treatment, and the establishment of healthcare facilities and advanced infrastructure. This is increasing the accessibility to advanced diagnostic methods for early diagnosis and treatment.
Among offerings, in 2024, the reagents & kits segment is expected to account for the largest share of the market. The commercial availability of a diverse range of diagnostic reagents & consumables, the availability of disease-specific test kits & assays, and growing awareness regarding early disease diagnosis are some of the major factors contributing to the growth of this segment. For instance, according to the World Health Organization (WHO) South East Asia, due to growing mortality rates, government-led, community-led, and corporate-led initiatives have been introduced to increase the awareness of disease diagnosis and increase the rate of testing, driving the demand for consumables. Some of the government-led initiatives include building a regional diagnostic strategy for ASEAN (Association of South East Asian Nations), collaborations with private stakeholders, directing funds to develop diagnostics infrastructure, and adopting hybrid testing network models that include centralized labs and point of care solutions. Similarly, educating individuals on the importance of screening & testing and driving the adoption of technology where the patients reside are some of the community-led initiatives, and corporate initiatives such as MNCs subsidizing costs of screening for employees, among others.
Among test types, in 2024, the laboratory tests segment is expected to account for the largest share of the market. Lab testing is a traditional approach in which the clinician takes a sample from the patient and sends it to the laboratory for processing and testing. This approach for molecular diagnostics is advantageous over other approaches due to higher accuracy and reliability, high sensitivity, and specificity, less expensive than point-of-care tests, higher availability of these tests in hospitals and healthcare institutions, and is also preferred by the patients, therefore, leading to the higher market share of this segment.
Among technologies, in 2024, the polymerase chain reaction (PCR) segment is expected to account for the largest share of the molecular diagnostics market in South East Asia. PCR technology is used to amplify small segments of DNA or RNA that are selected from the genome using a primer. Conventional PCR is used in many areas of study, including medical and diagnostic research, forensic studies and research, selective DNA isolation, amplification, and quantification of DNA. The large market share of the segment is attributed to its benefits, such as the ability to test for multi-drug resistance, test for multiple antibiotic genes with one sample, guide specific antimicrobial treatment while reducing the chance of developing drug resistance, and also to evaluate sensitivity to antimicrobials by quantitative analysis of the viral load after treatment. For instance, during the COVID-19 pandemic, Indonesia implemented a multifaceted testing strategy to detect and monitor COVID-19 cases. This included PCR (Polymerase Chain Reaction) testing in addition to rapid antigen testing, targeted testing, and surveillance testing. This was done to enhance its PCR capabilities.
Based on application, in 2024, the infectious disease segment is expected to account for the largest share of the molecular diagnostics market in South East Asia. Infectious diseases are transmissible or communicable illnesses that can spread quickly and become epidemics. It is crucial to quickly diagnose initial infections and prevent further spread through in vitro diagnosis. Some of the infections that are more prevalent in the South East Asian region include HIV, tuberculosis, malaria, arbovirus infection, dengue, chikungunya, and Japanese encephalitis. However, there is a continued concern over an influenza outbreak. Molecular diagnostics is widely used in infectious diseases as it includes rapid test results, facilitating detection of outbreaks, sensitivity, specificity, and identification of resistant organisms, and quantifiable correlation to disease severity, all of which contribute to the timely therapeutic clinical decisions and early infection control interventions, leading to the large market share of the segment.
Based on end users, in 2024, the hospitals & clinics segment is expected to account for the largest share of the molecular diagnostics market in South East Asia. Hospitals & clinics perform a wide range of tests to diagnose various medical conditions, including communicable and noncommunicable diseases, to ensure proper clinical planning of treatment and prevention. Hospitals provide access to diagnosis in urban and rural areas with large populations. The increasing prevalence of chronic diseases is driving the need for better healthcare options among patients. Apart from this, the increased number of hospitalizations due to various diseases requiring molecular diagnosis and the proliferation of hospitals and clinics in emerging countries.
The growth of this segment is primarily driven by the growing healthcare expenditure, growth in the adoption of molecular diagnostics, increasing prevalence of cancer and musculoskeletal diseases, rising disposable income, and improvements in the healthcare infrastructure. For this improvement, the Indonesian Ministry of Health, in February 2023, introduced the 2024 Digital Health Transformation Strategy to create an ‘Indonesia Sehat’ by collaborating with the entire ecosystem of health industry players through the SATUSEHAT platform. This is a digital health ecosystem platform that provides data connectivity, analysis, and services to support and integrate various health applications and resources. This will help the industry to effectively use upcoming and established technologies by interacting with one another and sharing data to make decisions about the delivery of care.
The key players profiled in the South East Asia molecular diagnostics market report are Sansure Biotech, Inc. (China), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), Xiamen Zeesan Biotech Co., Ltd (China), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.).
The report includes a competitive landscape based on an extensive assessment of the market based on offering, test type, application, technology, and end user. The report also provides insights into the presence of major market players and their key growth strategies in the last three to four years.
|
Particular |
Details |
|
Page No |
~220 |
|
Format |
|
|
Forecast Period |
2024-2031 |
|
Base Year |
2023 |
|
CAGR |
7.8% |
|
Market Size (Value) |
$891.87 million by 2031 |
|
Segments Covered |
By Offering
By Test Type
By Technology
By Application
By End User
|
|
Countries Covered |
Indonesia, Vietnam, Thailand, Singapore, Malaysia, and Rest of South East Asia |
|
Key Companies |
Sansure Biotech, Inc. (China), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), Xiamen Zeesan Biotech Co., Ltd (China), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.) |
This market study covers the market sizes & forecasts of the molecular diagnostics market in South East Asia based on offering, test type, technology, application, end user, and geography. This market study also provides the value analysis of various segments and subsegments of the South East Asia molecular diagnostics market at country levels.
The South East Asia molecular diagnostics market is projected to reach $891.87 million by 2031, at a CAGR of 7.8% during the forecast period.
The kits & reagents segment is expected to account for the largest share of the market in 2024. Factors such as the need for kits for the growing prevalence of diseases and the growing awareness of early diagnosis among people lead to the largest share of the segment.
The infectious diseases segment is expected to account for the largest share of the market in 2024. Infectious diseases are transmissible or communicable illnesses that spread quickly in some areas and become epidemics. It is crucial to quickly diagnose initial infections and prevent further spread through in vitro diagnosis. The growing prevalence of infectious diseases, the rising funds for infectious disease diagnostic research activities, and the sudden emergence of a pandemic are the factors attributed to the large market share of the segment.
The growth of the South East Asia molecular diagnostics market is driven by several factors, including the rising geriatric population, increasing prevalence of communicable & non-communicable diseases, technological advancements in molecular diagnostics, and rising health expenditure.
Furthermore, emerging medical tourism in South East Asian countries and increasing focus on companion diagnostics serve as major opportunities for the existing market players and new entrants in the South East Asia molecular diagnostics market.
The key players profiled in the South East Asia molecular diagnostics market report are Sansure Biotech, Inc. (China), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), Xiamen Zeesan Biotech Co., Ltd (China), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.).
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates