Resources
About Us
South East Asia IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics, Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology, Diabetes), Diagnostic Approach (Lab, POC) - Forecast to 2032
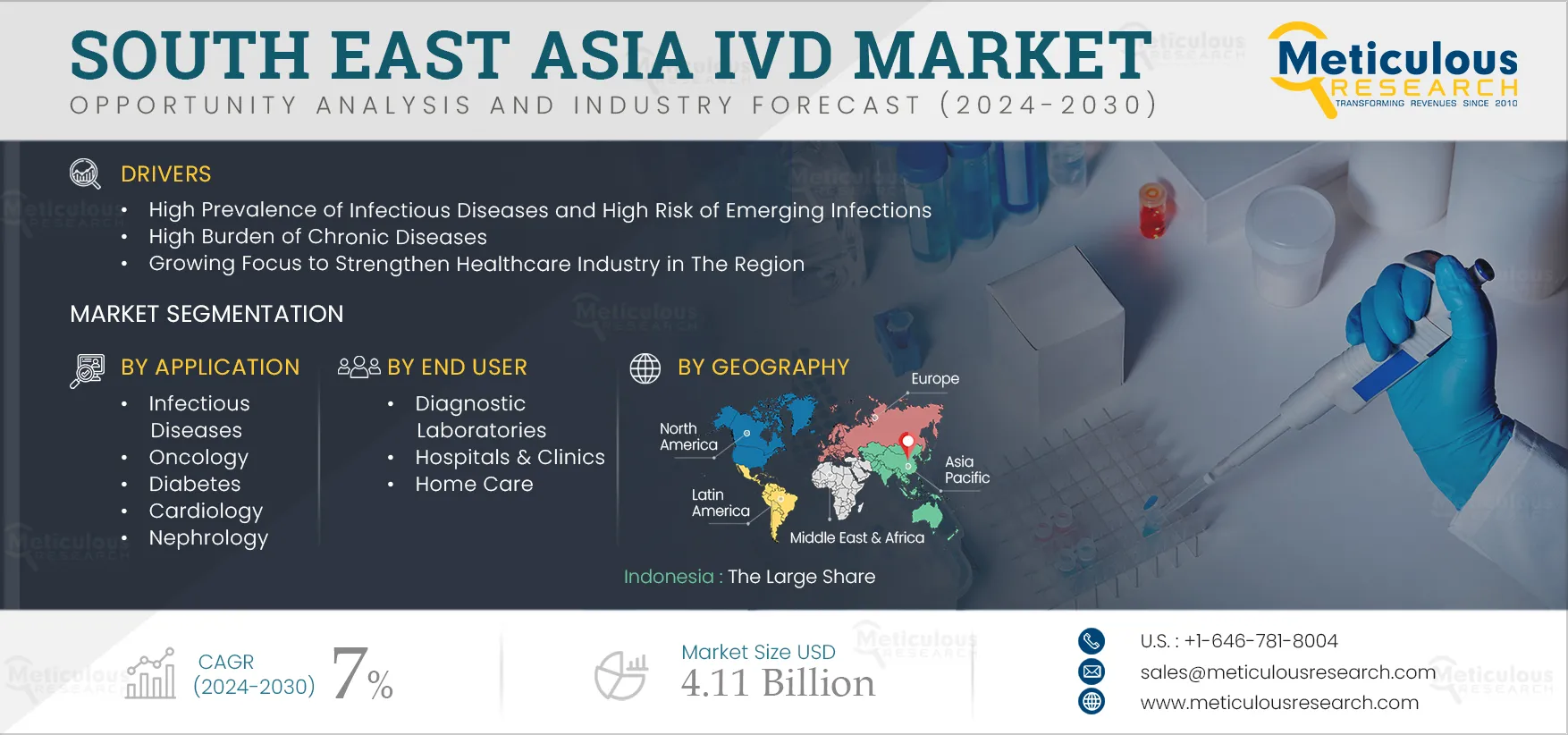
Report ID: MRHC - 104930 Pages: 200 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe South East Asia IVD Market is expected to record a CAGR of 7% from 2025 to 2032, reaching $4.11 billion by 2032. In vitro diagnostics (IVD) are tests performed on biological samples such as blood, saliva, and other tissues taken from the human body to detect a wide range of diseases. IVD tests screen biological samples to monitor individuals’ health and prevent or diagnose diseases. These consist of various tests based on techniques such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, polymerase chain reaction (PCR), isothermal nucleic acid amplification, and next-generation sequencing. These tests can be performed in laboratories, homes, and healthcare facilities.
The growth of this market is driven by the high prevalence of infectious diseases and the rising risk of emerging infections, the high burden of chronic diseases, and the growing focus on strengthening the healthcare industry in the region. Additionally, growing awareness regarding early disease diagnosis and growing medical tourism in the region are expected to create growth opportunities for the stakeholders in this market. However, the large import of tests and instruments and limited local manufacturing facilities restrain the growth of this market. Poor healthcare access in remote populations is a major challenge to market growth.
Despite the progress in healthcare, the region bears a significant burden of infectious diseases. The region has one of the largest HIV and tuberculosis burdens globally. South East Asia also has the third highest incidence of Malaria in all the WHO regions. Such high burden and incidence of infectious diseases create a demand for fast and efficient testing driving the IVD market in the region.
The South East Asian region is considered a high-risk hotspots and home for emerging infectious diseases (EID). The outbreaks of various novel influenza strain spillage of the Nipah virus were seen in these countries. This can be attributed to the highly diverse wildlife and changing demographics that bring the human population closer to the forests and natural wildlife reservoirs. These wildlife reservoirs are home to several pathogens and viruses. These viruses and pathogens are very likely to spill over and infect humans. Therefore, to prevent the further spread of these infections, there arises a need for early, rapid, and precise testing, driving the market growth.
Following are some recent infectious disease outbreaks in the region:
Click here to: Get Free Sample Pages of this Report
Development of healthcare infrastructure leads to increased accessibility of healthcare institutions, disease diagnostics & management, and improved overall health system. Compared to other developed regions, the healthcare system is underdeveloped in South East Asia. Looking at the high burden of infectious and chronic diseases in the region, governments and international organizations are focusing on developing healthcare in the region. For instance, in 2021, the U.S. government provided USD 40 million to the Association of Southeast Nations to strengthen the region's research and health system capacity.
Moreover, in 2021, the World Health Organization launched the ‘South-East Asia Regional Strategy for Primary Health Care: 2022-2032’ initiative. This initiative aims to accelerate the progress of all the countries in the South East Asia region towards universal health coverage (UHC) and health security and health-related sustainable development goals. Such initiatives are expected to strengthen the healthcare systems of the countries in the region, leading to increasing demand for in vitro diagnostic testing and ultimately driving market growth.
The segment's growth can be attributed to the initiatives by the government, private players, and institutions to support the development of molecular diagnostics and constant innovation and advancements in molecular technologies.
The rising adoption of point-of-care tests, the rising prevalence of chronic & infectious diseases, and the increasing launches of new disease-specific kits are the key factors contributing to the growing demand for consumables. Furthermore, the COVID-19 pandemic significantly boosted the demand for IVD reagents, assays, and kits in the region. For instance, in June 2020, WHO, in collaboration with the Indonesian Ministry of Health to enhance COVID-19 case detection and help strengthen Indonesia’s laboratories, supported the procurement and distribution of COVID-19 test kits.
The factors propelling the segment's growth include the high burden and increasing prevalence of cardiac disorders in the region, growing research on the development of cardiac biomarkers, and initiatives taken by organizations to increase awareness regarding cardiac diseases. The rate of deaths due to non-communicable diseases in South East Asia is high. One of the major diseases includes cardiovascular disease. According to the World Heart Federation, cardiovascular diseases account for around one-third of the total deaths in South East Asia, accounting for 4 million deaths per year.
The increasing demand for rapid and accurate testing is due to the large number of deaths caused by non-communicable diseases, including cardiovascular diseases, cancer, and diabetes, among others. Furthermore, the initiatives for strengthening healthcare infrastructure in the regions drive segment growth.
The increasing demand for rapid and accurate testing is due to the large number of deaths caused by non-communicable diseases, including cardiovascular diseases, cancer, and diabetes, among others. Furthermore, the initiatives for strengthening healthcare infrastructure in the regions drive segment growth. The large share of the segment can be attributed to the majority and complex diagnostic tests carried out by diagnostic laboratories, the high burden of infectious and chronic diseases, and the associations of hospitals with diagnostic laboratories. Hospitals are people's primary choice if symptoms are onset for any disease or disorder. Most regional hospitals outsource the test samples to diagnostic laboratories for precise test results.
In 2025, Indonesia is expected to dominate the South East Asia IVD market. The factors attributed to the large share are the high prevalence of infectious diseases, the increasing prevalence of chronic diseases, and the growing awareness regarding early disease diagnosis. For instance, new caser cases are estimated to increase to 645,436 by 2040 from 396,914 cases in 2020 (Source: GLOBCAN).
Key Players
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presence, and key strategic developments adopted by leading market players in this market in the last three to four years. The key players profiled in the South East Asia IVD market are Abbott Laboratories (U.S.), bioMérieux SA (France), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), PerkinElmer, Inc. (U.S.), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), and Sysmex Corporation. (U.S.).
Scope of the Report:
Key Questions Answered in the Report:
The South East Asia IVD market report covers market sizes & forecasts for reagents & kits, systems/instruments, and software & services. The South East Asia IVD market study includes the value analysis of various segments and subsegments of the South East Asia IVD market at the country level.
The South East Asia IVD market is projected to reach $4.11 billion by 2032, at a CAGR of 7% from 2025 to 2032.
The reagents & kits segment is estimated to account for the largest share of the South East Asia IVD market in 2025.
Based on technology, the molecular diagnostics segment is projected to gain more traction in the South East Asia IVD market.
Based on application, the cardiology segment is projected to gain more traction in the South East Asia IVD market.
The high growth of the South East Asia IVD market is mainly attributed to the high prevalence of infectious diseases and high risk of emerging infections, the high burden of chronic diseases, and the growing focus on strengthening the healthcare industry in the region. Additionally, growing awareness regarding early disease diagnosis and growing medical tourism in the region are expected to create growth opportunities for the stakeholders in this market.
The key players operating in the South East Asia IVD market are Abbott Laboratories (U.S.), bioMérieux SA (France), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), PerkinElmer, Inc. (U.S.), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), and Sysmex Corporation (U.S.).
Indonesia is expected to offer significant growth opportunities for the vendors in this market due to the high prevalence of infectious diseases, the increasing prevalence of chronic diseases, and the growing awareness regarding early disease diagnosis.
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates