Resources
About Us
Recombinant Coagulation Factors Market Size, Share, Forecast, & Trends Analysis by Type (Factor VIII, Factor IX), Source (CHO, HEK), Application (Hemophilia (Type A, Type B), Others), End User (Hospitals, CRO) - Global Forecast to 2031
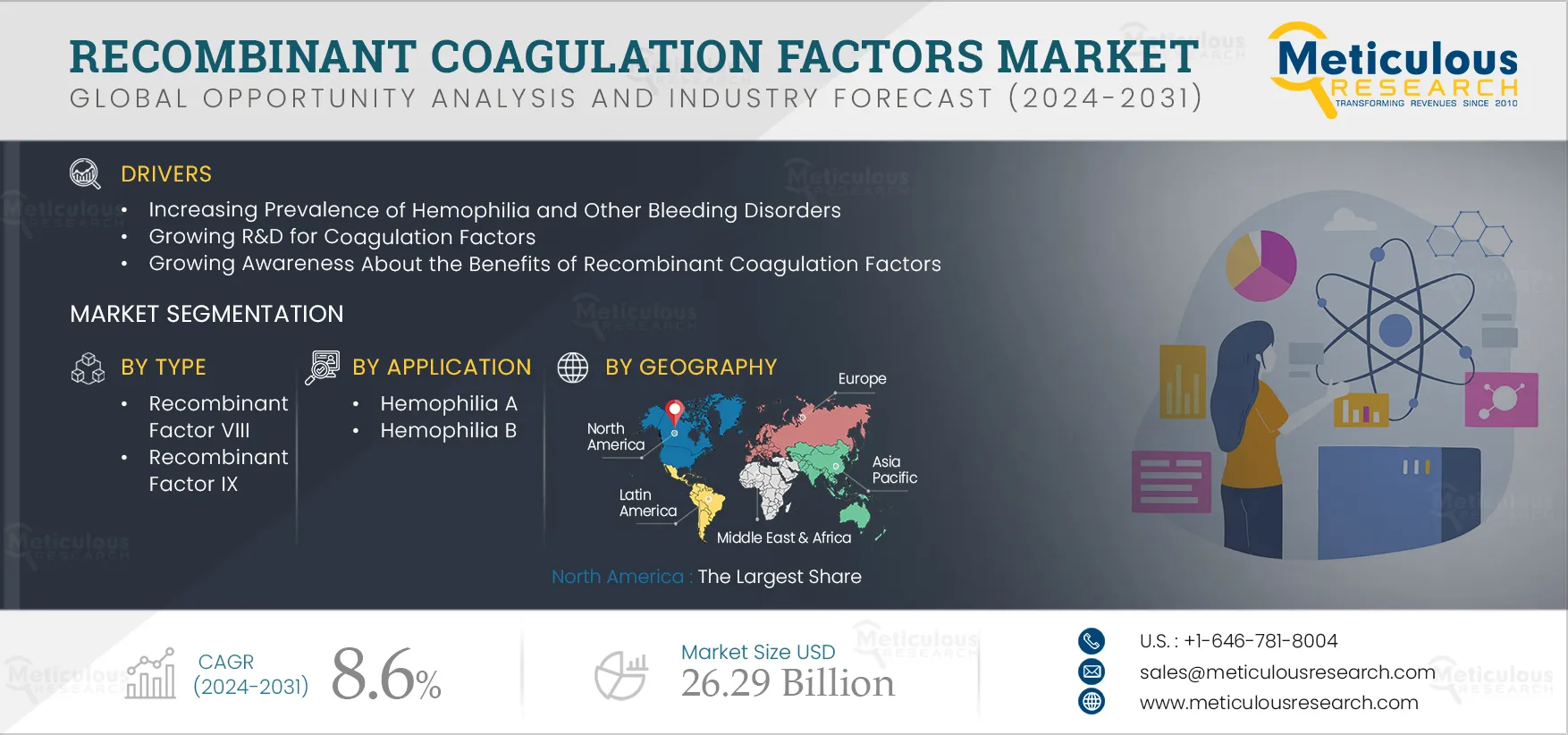
Report ID: MRHC - 104168 Pages: 180 Jul-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 48 Hours Download Free Sample ReportThe Recombinant Coagulation Factors Market is projected to reach $26.29 billion by 2031, at a CAGR of 8.6% from 2024 to 2031. Recombinant coagulation factors differ from plasma-derived factors as they are produced using recombinant DNA technology. This production method ensures a very low risk of blood-borne transmission of infectious diseases, effectively eliminating the threat of viruses such as hepatitis C and human immunodeficiency virus (HIV). As a result, recombinant coagulation factors are primarily used in treating bleeding disorders such as hemophilia.
The increasing prevalence of hemophilia and other bleeding disorders, growing R&D for coagulation factors, growing awareness about the benefits of the recombinant coagulation factors, and increasing prophylactic treatment for hemophilia across the globe are driving the growth of the recombinant coagulation factors market. However, the high cost of recombinant factors compared to plasma-derived factors and the limited accessibility & availability of recombinant clotting factors in developing & underdeveloped countries are expected to restrain the growth of this market.
Furthermore, untapped markets in emerging economies and rising healthcare expenditure are expected to create market growth opportunities. However, challenges in the production of recombinant coagulation factors, stringent regulatory requirements, lack of reimbursement in some countries, and lack of awareness among patients are some of the challenges impacting the market’s growth.
Hemophilia A and B are the most common severe hereditary hemorrhagic disorders. These disorders are caused due to coagulation factor VIII and factor IX protein deficiency. Patients with these disorders have prolonged bleeding with or without trauma, depending on the factor activity. According to National Center for Biotechnology Information (NCBI) data, in September 2022, the estimated frequency of hemophilia was about 1 in 10,000 live births, and the number of people worldwide living with hemophilia was about 4,00,000. Hemophilia A is more prevalent, about 80-85% of the total hemophilia population. Hemophilia A is present in 1 in 5,000 live male births, whereas hemophilia B is present in 1 in 30,000 live male births.
In order to tackle this disorder, coagulation factors, also known as clotting factors, are gaining popularity. The wide availability of recombinant factor VIII, viral inactivation, and better screening technology make the product and treatment safer. Thus, despite the availability of plasma-derived factor concentrates, about 75% of patients with hemophilia worldwide receive recombinant factor VIII products since they are much safer. Thus, the effectiveness of recombinant clotting factors products supports better treatment for hemophilic patients, driving the growth of this market.
Click here to: Get Free Sample Pages of this Report
Hemophilia, a treatable yet costly rare disease, receives reimbursement coverage in some countries such as China, the U.S., and European countries. The availability of reimbursement policies for the disease increases the accessibility to recombinant coagulation factors. From 2012, the Chinese Ministry of Health has prioritized 20 high-cost diseases (including hemophilia) to ease patients’ economic burden. Similarly, from 2021 onwards, the Medicaid and NC Health Choice programs in the U.S. began covering [coagulation factor VIIa (recombinant)-jncw] lyophilized powder for solution, for intravenous use (Sevenfact) for use in the Physician Administered Drug Program.
The half-life of the recombinant coagulation factors is referred to as the time taken by the factor to reduce the level by half. Many key players are trying to increase the half-life of recombinant coagulation factors to improve the effectiveness of treatment against Hemophilia. Extended half-life recombinant products are produced to improve the pharmacokinetic profile of factors to enable better hemostatic protection with a fewer number of injections in persons with hemophilia.
Pharmaceutical companies such as Novo Nordisk (Denmark), Baxter International Inc. (U.S.), and Bayer AG (Germany) are using the PEGylation method to extend the half-life of recombinant coagulation factors. The extended half-life of recombinant factors helps in reducing the number of injections, improving treatment adherence, enhancing protection against bleeding, and ultimately reducing economic burden.
Plasma-derived concentrates are made from human blood donated by healthy volunteers and screened for safety. Recombinant factor products are made in a laboratory using recombinant technology. These products are not made from human blood. Since recombinant factor products are manufactured in the laboratory, it avoids the risk of transmission of blood-borne viruses.
In the past, patients with bleeding disorders such as hemophilia relied on plasma concentrates for treatment. However, those treated with plasma concentrates derived from pooled plasma faced a significantly high risk of HIV infection due to the lack of effective screening for HIV in plasma and plasma donors. Historically, about 70-75% of patients with severe hemophilia were found to have contracted HIV as a result. This scenario prompted the development of recombinant coagulation factors, which provide a safer alternative to plasma-derived products by eliminating the risk of blood-borne transmission of infectious diseases. Therefore, the preference for recombinant factors over plasma-derived factors is expected to create growth opportunities for stakeholders in this market.
Based on type, the recombinant coagulation factors market is segmented into recombinant factor VIII, recombinant factor IX, and other types. In 2024, the recombinant factor VIII segment is expected to account for the largest share of 59.5% of this market. The large market share of this segment is attributed to advancements in recombinant technology, high demand for recombinant factor VIII due to a large patient pool of individuals with Hemophilia A, rising accessibility of treatment, and growing awareness about the benefits of recombinant coagulation factors. Factor VIII plays a crucial role in mitigating chronic damage and preventing fatal bleeding, making it a preferred treatment for bleeding disorders. These benefits contribute to the large market share of this segment.
Based on application, the recombinant coagulation factors market is segmented into hemophilia A, hemophilia B, and other applications. In 2024, the hemophilia A segment is expected to account for the largest share of 64.3% of the recombinant coagulation factors market. This segment’s large market share can be attributed to the larger number of patients with hemophilia A compared to hemophilia B, the increasing global prevalence of hemophilia, and supportive government initiatives aimed at enhancing treatment for hemophilia A. For instance, under India's National Health Mission, the government provides financial aid for the treatment of hemophilia A. Similarly, in the U.S., the National Hemophilia Program tracks patient data at national and regional levels to assess outcomes and improve care for individuals with hemophilia A.
Based on end user, the recombinant coagulation factors market is segmented into hospitals & clinics and clinical research laboratories. In 2024, the hospitals & clinics segment is expected to account for the largest share of the recombinant coagulation factors market. The large market share of this segment is attributed to the increased adoption of recombinant coagulation factors in hospitals for the treatment of coagulation disorders, rising healthcare expenditure, and rising awareness among healthcare professionals. Furthermore, patients have access to reimbursement policies and government schemes designed to support the treatment of bleeding disorders. Many hospitals and clinics are forming partnerships with key players or other healthcare facilities to enhance treatment accessibility. For instance, in May 2023, the Amrita Hemophilia Treatment Center (India) partnered with the Manchester Hemophilia Treatment Center (U.S.) through a twinning program aimed at improving hemophilia care in India.
In 2024, North America is expected to account for the largest share of the 43.4% of recombinant coagulation factors market. North America’s significant market share can be attributed to the increasing prevalence of hemophilia and bleeding disorders, rising accessibility and affordability for the treatment, supportive government initiatives aimed at enhancing treatment, the proliferation of treatment & research centers for hemophilia, and the increasing number of awareness programs for hemophilia. According to the Centers for Disease Control and Prevention, the hemophilia program is the largest in the CDC’s Division of Blood Disorders, working towards creating awareness about hemophilia causes, symptoms, and treatment.
However, the market in Asia-Pacific is projected to register the highest CAGR of 10.3% during the forecast period. The growth of this market is driven by the increasing healthcare investments in developing countries like China and India and the rising preference for recombinant technology due to its advantages over traditional methods.
The report offers a competitive landscape based on an extensive assessment of the leading players’ product offerings and geographic presence and the key growth strategies adopted by them over the past few years. The key players profiled in the recombinant coagulation factors market report are Baxter International Inc. (U.S.), Grifols, S.A. (Spain), CSL Limited (Australia), Octapharma AG (Switzerland), Novo Nordisk A/S (Denmark), Biogen Inc. (U.S.), Bayer AG (Germany), Kedrion S.p.A. (Italy), Emergent BioSolutions (U.S.), and Pfizer Inc. (U.S.).
|
Particulars |
Details |
|
Number of Pages |
180 |
|
Format |
|
|
Forecast Period |
2024-2031 |
|
Base Year |
2022 |
|
CAGR |
8.6% |
|
Estimated Market Size (Value) |
$26.29 billion by 2031 |
|
Segments Covered |
By Type
By Source
By Application
By End User
|
|
Countries Covered |
North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Netherlands, Sweden, and Rest of Europe), Asia-Pacific (China, Japan, India, Australia, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, Rest of Latin America), and Middle East & Africa |
|
Key Companies |
Baxter International Inc. (U.S.), Grifols, S.A. (Spain), CSL Limited (Australia), Octapharma AG (Switzerland), Novo Nordisk A/S (Denmark), Biogen Inc. (U.S.), Bayer AG (Germany), Kedrion S.p.A. (Italy), Emergent BioSolutions (U.S.), and Pfizer Inc. (U.S.). |
This study offers a detailed assessment of the recombinant coagulation factors market and analyzes the market sizes & forecasts based on type, source, application, and end user. This report also involves the value analysis of various segments and subsegments of the recombinant coagulation factors market at the regional and country levels.
The recombinant coagulation factors market is projected to reach $26.29 billion by 2031, at a CAGR of 8.6% during the forecast period.
Based on type, in 2024, the recombinant factor VIII segment is expected to account for the largest share of the market due to increased demand for the treatment of hemophilia A and advancement in recombinant technology.
Based on application, the hemophilia A segment is projected to register the highest CAGR during the forecast period. The growth of this segment is driven by the increasing incidence of hemophilia and the rise in diagnosis rates, leading to the increased adoption of recombinant coagulation factors.
The increasing prevalence of hemophilia and other bleeding disorders, growing R&D for coagulation factors, growing awareness about the benefits of the recombinant coagulation factors, and increasing prophylactic treatment for hemophilia across the globe are driving the growth of the recombinant coagulation factors market. Furthermore, untapped markets in emerging economies and rising healthcare expenditure are expected to create market growth opportunities.
The key players profiled in the recombinant coagulation factors market study are Baxter International Inc. (U.S.), Grifols, S.A. (Spain), CSL Limited (Australia), Octapharma AG (Switzerland), Novo Nordisk A/S (Denmark), Biogen Inc. (U.S.), Bayer AG (Germany), Kedrion S.p.A. (Italy), Emergent BioSolutions (U.S.), and Pfizer Inc. (U.S.).
Emerging economies such as China and India are projected to offer significant growth opportunities to the market players due to rising healthcare expenditure, large patient volume suffering from hemophilia, and the high market expansion potential in these countries.
























Published Date: Jul-2020
Published Date: Jul-2022
Published Date: Sep-2022
Published Date: Sep-2022
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates