Resources
About Us
Real-world Evidence Solutions Market Size, Share, Forecast, & Trends Analysis by Component (Datasets [Clinical, Claims, Pharmacy], Services) Application (Market Access, Drug Development & Approvals, PMS), and End User - Global Forecast to 2031
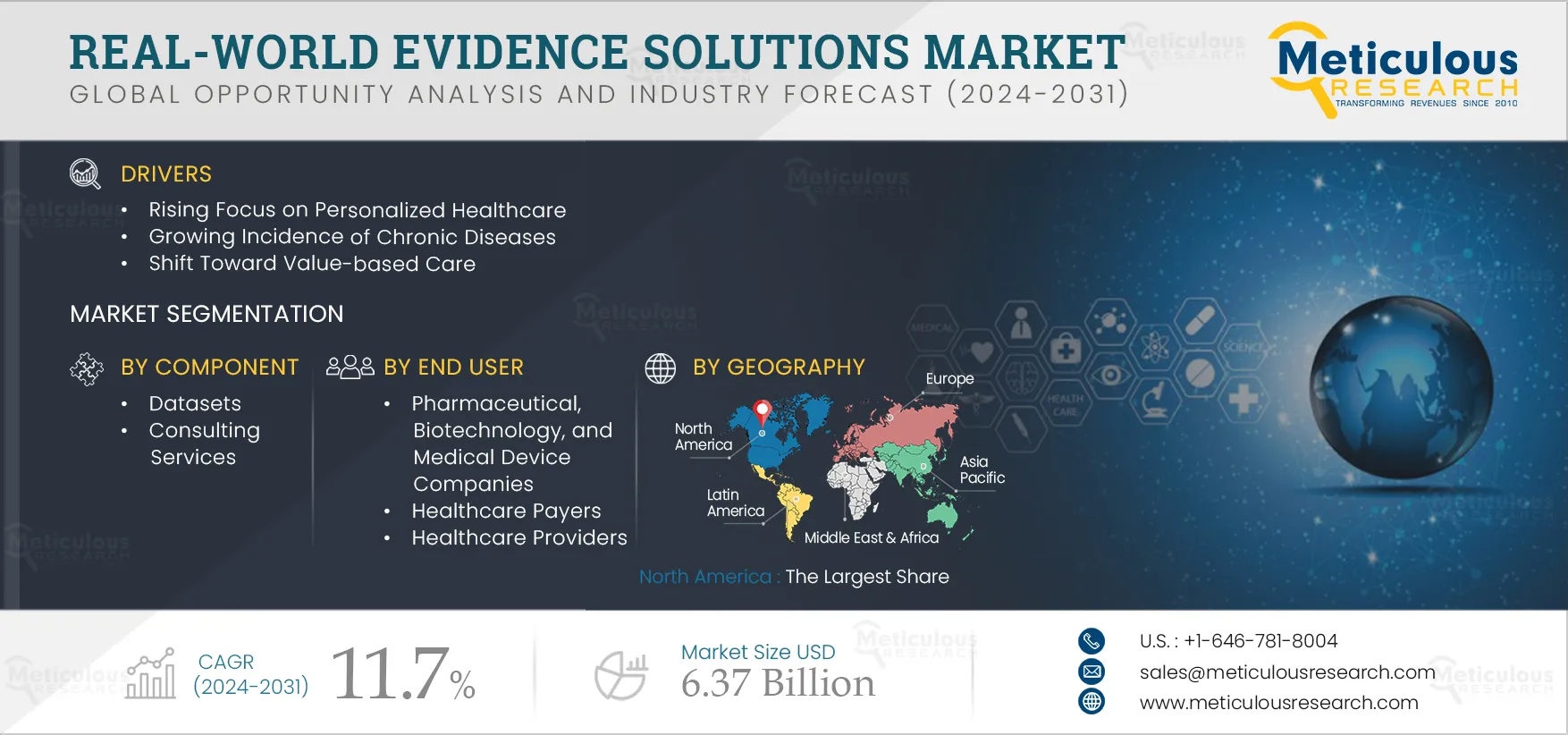
Report ID: MRHC - 104253 Pages: 254 Aug-2024 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportThe growth of the real-world evidence solutions market is driven by the rising incidence of chronic diseases, delays in drug development and the consequent increase in development costs, the rising focus on personalized healthcare, a shift toward value-based care, and the growing adoption of real-world evidence solutions in drug development and commercialization. Furthermore, emerging economies and an increasing focus on end-to-end RWE services are expected to offer significant growth opportunities for players operating in the real-world evidence solutions market.
Personalized medicine is one of the emerging approaches to improve disease treatment and prevention, considering individuals’ variability in genes, environment, and lifestyles. However, this approach can be challenging, as designing an effective treatment plan for a medically complex patient requires a thorough analysis of a large volume of data. Providers may face difficulties due to limited resources, which can hinder their ability to extract and utilize valuable information effectively. The use of real-world evidence accelerates the transition to precision medicine.
It enables researchers to extend beyond the limitations of traditional clinical trials by utilizing real-world data gathered during patients' routine clinical care. This real-world information allows researchers to identify which medications are best suited for individual patients, leading to more personalized therapies and improved outcomes. The increasing approvals for personalized medicines are expected to increase the demand for real-world evidence solutions. According to the Personalized Medicine Coalition (PMC), in the U.S. in 2022, 34% of FDA-approved medicines were classified as personalized medicine, up from 25% in 2019.
Click here to: Get Free Sample Pages of this Report
With the shift towards personalized healthcare and the emergence of rare diseases, healthcare, and research organizations are facing challenges such as high costs and lengthy timelines for delivering medicines to patients as the development of new pharmaceutical entities is time-consuming, extremely costly, and of high-risk with fewer chances of successful outcomes. For instance, it is estimated that the average cost of drug development ranges from USD 43.4 million to USD 4.2 billion (Source: WHO 2022 report). Pharmaceutical companies are also confronting rising clinical trial costs and are seeking ways to reduce these expenses to expedite drug development. Real-world evidence (RWE) solutions offer a promising approach by simplifying access, alleviating burdens, and providing deeper insights into the real-world use of medicines. The growing use of real-world data helps to accelerate drug development, streamline approvals, and lower overall costs.
Enhancements in data management and data integration have improved the speed & quality of drug discovery and clinical trials. Artificial intelligence (AI) is employed in real-world data (RWD) to enhance data anomaly detection, standardization, and quality checking at the pre-processing stage. AI is expected to provide pharmaceutical and biotech companies with the ability to increase meaningful RWE output, decrease the time required to derive valuable insights and maximize the use of available data sources. RWE technology platforms that offer advanced data processing, analysis, and outcomes present an unparalleled opportunity to leverage these computing advancements. When integrated into a comprehensive RWE strategy, AI innovations can enhance drug development, improve patient treatment, access, and drive new business opportunities. AI-driven analytics and automation provide access to crucial insights from historical clinical trial data and real-world evidence (RWE), expanding end-to-end clinical trial capabilities.
Enhancements in data management and integration have improved the speed & quality of drug discovery and clinical trials. Artificial intelligence (AI) enhances real-world data (RWD) by improving anomaly detection, standardization, and quality checking during pre-processing. AI is expected to boost meaningful RWE output, shorten insight generation time, and optimize data use for pharma and biotech companies. RWE platforms with advanced AI-driven data processing and analysis provide a unique opportunity to leverage these technologies. As part of a comprehensive RWE strategy, AI can accelerate drug development, enhance patient treatment, access, and create new business opportunities. AI-integrated analytics also offer critical insights from historical clinical trial data and RWE, expanding clinical trial capabilities.
The healthcare industry has witnessed tremendous change in the last few years, with a shift toward value-based care in developed economies spurred by healthcare organizations’ efforts to reduce costs, improve outcomes, and enhance returns on investment. Various organizations are focusing on population health and driving improved outcomes through value-based care delivery models. Companies must possess strong evidence lifecycle management capabilities to prove and articulate their value propositions. An end-to-end approach to leveraging data, evidence, and knowledge assets of a life sciences organization enables insight-driven decision-making from R&D through commercialization. To leverage the opportunities offered by RWD and RWE, various players are providing end-to-end and late-phase services, such as study planning, protocol development, and clinical study management & reporting. The key players offering end-to-end RWE services are Medstreaming (U.S.), Oracle Corporation (U.S.), SAS Institute Inc. (U.S.), Sciformix Corporation (U.S.), and PAREXEL International Corporation (U.S.).
Based on offering, the global real-world evidence (RWE) solutions market is segmented into datasets and consulting & analytics. In 2024, the datasets segment is expected to account for the largest share of 53% of real-world evidence (RWE) solutions. Real-world data (RWD) are collected from diverse sources related to patient outcomes in real-world settings. This data is often used in retrospective studies to generate real-world evidence (RWE). RWE offers valuable insights into unmet needs, as well as the clinical and economic impacts on patients and healthcare systems. It helps determine outcomes based on larger data samples, reduces costs, and enhances the efficiency of clinical trials. The shift towards personalized medicine, which increasingly relies on extensive datasets to identify patient subgroups and tailor treatments, further drives the demand for larger datasets. This approach is particularly beneficial for diseases like cancer, thus contributing significantly to the growth of this segment.
However, the consulting & analytics segment is estimated to register the highest CAGR of 15.2% during the forecast period. Consulting services encompass observational or prospective studies that involve the primary collection of data from individuals over time as their characteristics evolve. Analytic services include online platforms and software that generate real-world evidence (RWE). As data complexity grows, the demand for consulting and analytics services is rising. Technological advancements, such as wearables, electronic health records, and mobile applications, are driving an increase in data volume. Consequently, there is a growing need for consulting and analytics services to clean, manage, and analyze this data effectively.
Based on application, the global real-world evidence (RWE) solutions market is segmented into drug development & approvals, medical device development & approvals, market access & reimbursement/coverage decisions, post-market surveillance, and clinical & regulatory decision-making. In 2024, the market access & reimbursement/coverage decisions segment is expected to account for the largest share of 53.4% of the real-world evidence (RWE) solutions market. Real-world evidence (RWE) offers payers valuable insights into how treatments perform in everyday settings, aiding their decisions on medication coverage and reimbursement, which drives adoption. Monitoring drug safety and effectiveness is crucial for sustaining market access. RWE solutions assess pharmaceutical products' performance across diverse patient populations, considering real-world factors such as adherence, comorbidities, and concomitant medications, thereby reducing the risk of drug trial failures. Additionally, the FDA utilizes RWE to evaluate and monitor the post-market safety of approved drugs, contributing significantly to the growth of this segment.
Based on end user, the global real-world evidence (RWE) solutions market is segmented into pharmaceutical, biotechnology, and medical device companies, healthcare payers, healthcare providers, and other end users. In 2024, the pharmaceutical, biotechnology, and medical device companies’ segment is expected to account for the largest share of 39.1% of the real-world evidence (RWE) solutions market. This segment’s large market share is attributed to the increasing importance of RWE studies in drug development & approvals and the growing need to avoid drug recalls and assess drug performance in real-world settings. According to the Congressional Budget Office, the average cost of drug development ranges from USD 1 billion to over USD 2 billion, depending on the type of drug. These high costs present significant technical, regulatory, and economic challenges for pharmaceutical R&D pipelines. Consequently, pharmaceutical companies leverage RWE solutions to assess real-world data (RWD) and understand the potential risks and benefits of drugs, thereby optimizing drug design and development.
Based on geography, the global real-world evidence solutions market is segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. In 2024, North America is expected to account for the largest share of 48.9% of the real-world evidence (RWE) solutions market. Several factors, including the rising prevalence of chronic diseases, stringent drug approval regulations, the availability of electronic datasets, advancements in the healthcare industry, and the growing volume of big data in healthcare, drive the significant market share in this region. Additionally, supportive government initiatives for deploying RWE solutions further bolster this market share.
In August 2023, the U.S. FDA released a report titled "Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision-Making for Drug and Biological Products." This report outlines the FDA's framework for incorporating RWE solutions into regulatory decision-making processes.
However, Asia-Pacific is slated to register the highest growth rate of 11.2% during the forecast period. Countries like Japan and Taiwan have started integrating real-world evidence (RWE) into their healthcare systems through initiatives such as Japan's "Rational Medicine" program. This effort aims to make the Japanese healthcare system more patient-centric and evidence-based, driving market growth in the region. Additionally, supportive government initiatives in these countries are further fueling market expansion.
The report offers a competitive analysis based on an extensive assessment of the product portfolios and geographic presence of leading market players and the key growth strategies adopted by them over the past three to four years. Some of the key players operating in the real-world evidence solutions market are IQVIA Holdings Inc. (U.S.), Elevance Health, Inc. (U.S.), ICON plc (Ireland), Clinigen Group plc (U.K.), Cognizant Technology Solutions Corporation (U.S.), Revvity, Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Oracle Corporation (U.S.), SAS Institute Inc. (U.S.), Parexel International Corporation (U.S.), and HealthVerity, Inc. (U.S.).
|
Particulars |
Details |
|
Number of Pages |
254 |
|
Format |
|
|
Forecast Period |
2024–2031 |
|
Base Year |
2023 |
|
CAGR |
11.7% |
|
Market Size |
USD 6.37 Billion by 2031 |
|
Segments Covered |
By Component
By Application
By End User
|
|
Countries Covered |
North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, Belgium, and Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Taiwan, Singapore, Australia, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and Middle East & Africa |
|
Key Companies |
IQVIA Holdings Inc. (U.S.), Elevance Health, Inc. (U.S.), ICON plc (Ireland), Clinigen Group plc (U.K.), Cognizant Technology Solutions Corporation (U.S.), Revvity, Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Oracle Corporation (U.S.), SAS Institute Inc. (U.S.), Parexel International Corporation (U.S.), and HealthVerity, Inc. (U.S.) |
The global real-world evidence solutions market is expected to reach $6.37 Billion by 2031, at a CAGR of 11.7% from 2024 to 2031.
The real-world evidence solutions market study focuses on market assessment and opportunity analysis through the sales of real-world evidence solutions across different regions and countries across different market segments. This study is also focused on competitive analysis for RWE solutions based on an extensive assessment of the leading players’ product portfolios, geographic presence, and key growth strategies.
In 2024, the datasets segment is projected to hold the largest share of the global real-world evidence (RWE) solutions market. The increasing demand for insights into epidemiology, compliance, adherence, and costs within real-world settings drives this significant market share. Additionally, the growing volume of medical data generated by hospitals, the rising reliance of outcome-based studies on real-world data (RWD), and the heightened demand for drug safety information among healthcare payers, regulatory bodies, and providers contribute to this segment's dominance.
The growth of the real-world evidence solutions market is driven by the growing incidence of chronic diseases, delays in drug development and the consequent increase in development costs, the rising focus on personalized healthcare, a shift toward value-based care, and the growing adoption of real-world evidence solutions in drug development and commercialization. Furthermore, emerging economies and a rising focus on end-to-end RWE services are expected to offer significant growth opportunities for players operating in the real-world evidence solutions market.
The key players operating in the real-world evidence solutions market are global IQVIA Holdings Inc. (U.S.), Elevance Health, Inc. (U.S.), ICON plc (Ireland), Clinigen Group plc (U.K.), Cognizant Technology Solutions Corporation (U.S.), Revvity, Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Oracle Corporation (U.S.), SAS Institute Inc. (U.S.), Parexel International Corporation (U.S.), and HealthVerity, Inc. (U.S.).
Asia-Pacific is projected to register the highest CAGR of 11.2% during the forecast period. Countries like Japan and Taiwan have begun incorporating real-world evidence into their healthcare systems owing to the “Rational Medicine” initiative, which is driving market growth in the region. The initiative aims to make the Japanese healthcare system more patient-centric and evidence-based.
1. Introduction
1.1. Market Definition & Scope
1.2. Market Ecosystem
1.3. Currency & Limitations
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Process
2.2. Data Collection & Validation Process
2.2.1. Secondary Research
2.2.2. Primary Research/Interviews with Key Opinion Leaders from the Industry
2.3. Market Sizing & Forecasting
2.3.1. Market Size Estimation Approach
2.3.2. Growth Forecast Approach
2.3.3. Market Share Analysis
2.4. Assumptions For the Study
3. Executive Summary
4. Market Insights
4.1. Overview
4.2. Factors Affecting Market Growth
4.2.1. Market Dynamics
4.2.1.1. Drivers
4.2.1.1.1. Rising Focus on Personalized Healthcare
4.2.1.1.2. Delays in Drug Development and the Consequent Increase in Development Costs
4.2.1.1.3. Growing Incidence of Chronic Diseases
4.2.1.1.4. Shift toward Value-based Care
4.2.1.1.5. Rapidly Growing Big Data in the Healthcare Sector
4.2.1.2. Restraints
4.2.1.2.1. Reluctance to Rely on Real-world Studies
4.2.1.3. Opportunities
4.2.1.3.1. Emerging economies
4.2.1.3.2. Rising Focus on End-to-End RWE Services
4.2.1.4. Challenges
4.2.1.4.1. Lack of Standardized Methodologies for Developing RWE
4.2.2. Factors Analysis
4.3. Key Market Trends
4.3.1. The Incorporation of Artificial Intelligence (AI) in the RWE Industry
4.3.1.1. The Adoption of AI Technologies is Growing in the Following Areas:
4.3.1.1.1. Clinical Trials Design
4.3.1.1.2. Modeling and Forecasting Patient Enrichment & Recruitment
4.3.1.1.3. Selecting Investigator Site
4.3.1.1.4. Patient Monitoring & Managing and Medication Adherence & Retention
4.3.1.1.5. AI-enabled Clinical Trial Analytics
4.3.1.1.6. Outsourcing Essential AI Solutions via Strategic Partnerships & Collaborations
4.3.1.1.7. Market Access Using AI Solutions
4.3.1.1.8. Post-market Surveillance Using AI Solutions
4.3.2. Growing Adoption of RWE In Drug Development and Commercialization
4.3.3. Rising Number of Consolidations
4.3.4. Improved Patient Outcomes and Value Creation from Real-World Evidence
4.4. Case Studies For AI-based RWE Solutions
4.4.1. AI-based RWE Solutions for Medical Device Post-market Studies - Huma.AI (U.S.)
4.4.2. AI-enabled RWE Solutions to Support Clinical Decision-making in Drug Research & Development - Owkin, Inc. (France)
4.4.3. AI-integrated RWE Solutions for Market Access - Medaffcon Oy (Finland)
4.4.4. Leveraging Real-world Data Insights Using AI & Machine Learning Algorithms - Aetion, Inc. (U.S.) & Quinten Health (France)
4.4.5. AI-powered RWE Platform to Track Adverse Events - Data2Life (Israel)
4.4.6. AI-powered RWE Solutions to Predict Patients' Health at Critical Care Units - PHASTAR (U.K.)
4.5. Regulatory Analysis
4.5.1. Overview
4.5.2. North America
4.5.3. Europe
4.5.4. Asia-Pacific
4.5.5. Rest of the World
4.6. Pricing Models (EMR/Genomic/Integrated Datasets)
4.6.1. Overview
4.6.2. Pay Per Patient Record (Volume-based Pricing)
4.6.3. Pay Per Usage (Value-based Pricing)
4.6.4. Annual Subscription
5. Real-World Evidence (RWE) Solutions Market Assessment—by Component
5.1. Overview
5.2. Datasets
5.2.1. Disparate Datasets
5.2.1.1. EMR/EHR/Clinical Data
5.2.1.2. Claims & Billing Data
5.2.1.3. Pharmacy Data
5.2.1.4. Product/Disease Registries Data
5.2.1.5. Genomics Data
5.2.1.6. Other Disparate Datasets
5.2.2. Integrated Datasets
5.3. Consulting & Analytics
6. Real-World Evidence Solutions Market Assessment—by Application
6.1. Overview
6.2. Market Access & Reimbursement/Coverage Decisions
6.3. Drug Development & Approvals
6.3.1. Oncology
6.3.2. Neurology
6.3.3. Immunology
6.3.4. Cardiovascular Diseases
6.3.5. Other Therapeutic Areas
6.4. Post-market Surveillance
6.5. Medical Device Development & Approvals
6.6. Other Applications
7. Real-World Evidence (RWE) Solutions Market Assessment—by End User
7.1. Overview
7.2. Pharmaceutical, Biotechnology, and Medical Device Companies
7.3. Healthcare Payers
7.4. Healthcare Providers
7.5. Other End Users
8. Real-world Evidence Solutions Market Assessment—by geography
8.1. Overview
8.2. North America
8.2.1. U.S.
8.2.2. Canada
8.3. Europe
8.3.1. U.K.
8.3.2. Germany
8.3.3. France
8.3.4. Italy
8.3.5. Spain
8.3.6. Switzerland
8.3.7. Belgium
8.3.8. Rest of Europe
8.4. Asia-Pacific
8.4.1. Japan
8.4.2. China
8.4.3. India
8.4.4. South Korea
8.4.5. Australia
8.4.6. Taiwan
8.4.7. Singapore
8.4.8. Rest of Asia-Pacific
8.5. Latin America
8.5.1. Brazil
8.5.2. Mexico
8.5.3. Rest of Latin America
8.6. Middle East & Africa
9. Competitive Landscape
9.1. Introduction
9.2. Key Growth Strategies
9.3. Competitive Benchmarking
9.4. Competitive Dashboard
9.4.1. Industry Leaders
9.4.2. Market Differentiators
9.4.3. Vanguards
9.4.4. Emerging Companies
9.5. Market Share Analysis
9.5.1. IQVIA Holdings Inc. (U.S.)
9.5.2. Icon plc (IRELAND)
9.5.3. Thermo Fisher Scientific Inc. (U.S.)
10. Company Profiles
10.1. IQVIA Holdings, Inc.
10.2. Icon plc
10.3. Thermo Fisher Scientific Inc.
10.4. F. Hoffmann-La Roche Ltd
10.5. Oracle Corporation
10.6. Elevance Health, Inc.
10.7. Clinigen Group Plc
10.8. Cognizant Technology Solutions Corporation
10.9. Revvity, Inc.
10.10. SAS Institute Inc.
10.11. Parexel International Corporation
10.12. Healthverity, Inc.
11. Appendix
11.1. Available Customization
11.2. Related Reports
List of Tables
Table 1 Total Cost Per Study, by Phase and Therapeutic Area (USD Million)
Table 2 Sources of Healthcare Data
Table 3 Number of People aged 65 years or Over, by Region, 2019 vs. 2050 (Million)
Table 4 Strategic Developments of Key Players Related to the Integration of AI into RWE Solutions
Table 5 Real-world Evidence Status in Latin America
Table 6 Observed Indicative Prices of Real-world Data by Type (USD Per Patient Record)
Table 7 Global Real-world Solutions Market, by Component, 2022—2031 (USD Million)
Table 8 Global RWE Datasets Market, by Type, 2022-2031 (USD Million)
Table 9 Global RWE Datasets Market, by Country/Region, 2022–2031 (USD Million)
Table 10 Global RWE Disparate Datasets Market, by Type, 2022-2031 (USD Million)
Table 11 Global RWE Disparate Datasets Market, by Country/Region, 2022–2031 (USD Million)
Table 12 Global EMR/EHR/Clinical Data Market, by Country/Region, 2022–2031 (USD Million)
Table 13 Global Claims and Billing Data Market, by Country/Region, 2022–2031 (USD Million)
Table 14 Global Pharmacy Data Market, by Country/Region, 2022–2031 (USD Million)
Table 15 Global Product/Disease Registries Data Market, by Country/Region, 2022–2031 (USD Million)
Table 16 Global Genomics Data Market, by Country/Region, 2022–2031 (USD Million)
Table 17 Global Other Disparate Datasets Market, by Country/Region, 2022–2031 (USD Million)
Table 18 Global RWE Integrated Datasets Market, by Country/Region, 2022–2031 (USD Million)
Table 19 Global RWE Consulting & Analytics market, by Country/Region, 2022–2031 (USD Million)
Table 20 Global Real-world Solutions Market, by Application, 2022–2031 (USD Million)
Table 21 Global RWE Solutions Market for Market Access & Reimbursement/Coverage Decisions, by Country/Region, 2022–2031 (USD Million)
Table 22 Global RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 23 Global RWE Solutions Market for Drug Development & Approvals, by Country/Region, 2022–2031 (USD Million)
Table 24 Global RWE Solutions Market for Oncology, by Country/Region, 2022–2031 (USD Million)
Table 25 Global RWE Solutions Market for Neurology, by Country/Region, 2022–2031 (USD Million)
Table 26 Global RWE Solutions Market for Immunology, by Country/Region, 2022–2031 (USD Million)
Table 27 Estimated Medical Costs Associated with Cardiovascular Diseases (2020–2030) (USD Billion)
Table 28 Global RWE Solutions Market for Cardiovascular Diseases, by Country/Region, 2022–2031 (USD Million)
Table 29 Global RWE Solutions Market for Other Therapeutic Areas, by Country/Region, 2022–2031 (USD Million)
Table 30 Global RWE Solutions Market for Post-market Surveillance, by Country/Region, 2022–2031 (USD Million)
Table 31 Global RWE Solutions Market for Medical Device Development & Approvals, by Country/Region, 2022–2031 (USD Million)
Table 32 Global RWE Solutions Market for Other Applications, by Country/Region, 2022–2031 (USD Million)
Table 33 Global Real-world Solutions Market, by End User, 2022—2031 (USD Million)
Table 34 Global RWE Solutions Market for Pharmaceutical, Biotechnology, and Medical Device Companies, by Country/Region, 2022–2031 (USD Million)
Table 35 Global RWE Solutions Market for Healthcare Payers, by Country/Region, 2022–2031 (USD Million)
Table 36 Global RWE Solutions Market for Healthcare Providers, by Country/Region, 2022–2031 (USD Million)
Table 37 Global RWE Solutions Market for Other End Users, by Country/Region, 2022–2031 (USD Million)
Table 38 Global RWE Solutions Market, by Country/Region, 2022–2031 (USD Million)
Table 39 North America: RWE Solutions Market, by Country, 2022-2031 (USD Million)
Table 40 North America: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 41 North America: RWE Datasets Market, By Type, 2022–2031 (USD Million)
Table 42 North America: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 43 North America: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 44 North America: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 45 North America: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 46 U.S.: RWE Solutions Market, by component, 2022–2031 (USD Million)
Table 47 U.S.: RWE Datasets Market, By Type, 2022–2031 (USD Million)
Table 48 U.S.: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 49 U.S.: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 50 U.S.: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 51 U.S.: RWE Solution, by End User, 2022–2031 (USD Million)
Table 52 Canada: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 53 Canada: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 54 Canada: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 55 Canada: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 56 Canada: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 57 Canada: RWE Solution, by End User, 2022–2031 (USD Million)
Table 58 EUROPE: RWE solutions Market, by Country/Region, 2022-2031 (USD Million)
Table 59 Europe: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 60 Europe: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 61 Europe: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 62 Europe: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 63 Europe: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 64 Europe: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 65 U.K.: Conferences and Workshops on Real-world Evidence Solutions
Table 66 U.K.: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 67 U.K.: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 68 U.K.: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 69 U.K.: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 70 U.K.: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 71 U.K.: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 72 Conferences, Symposia, and Workshops in Germany
Table 73 Germany: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 74 Germany: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 75 Germany: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 76 Germany: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 77 Germany: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 78 Germany: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 79 France: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 80 France: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 81 France: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 82 France: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 83 France: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 84 France: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 85 Italy: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 86 Italy: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 87 Italy: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 88 Italy: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 89 Italy: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 90 Italy: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 91 Spain: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 92 Spain: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 93 Spain: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 94 Spain: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 95 Spain: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 96 Spain: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 97 Switzerland: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 98 Switzerland: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 99 Switzerland: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 100 Switzerland: RWE solutions Market, by Application, 2022–2031 (USD Million)
Table 101 Switzerland: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 102 Switzerland: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 103 Belgium: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 104 Belgium: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 105 Belgium: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 106 Belgium: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 107 Belgium: RWE Solutions Market For Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 108 Belgium: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 109 Rest of Europe: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 110 Rest of Europe: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 111 Rest of Europe: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 112 Rest of Europe: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 113 Rest of Europe: RWE Solutions Market for Drug Development & Approvals, BY Therapeutic Area, 2022–2031 (USD Million)
Table 114 Rest of Europe: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 115 Asia-Pacific: RWE Solutions Market, by Country/Region, 2022–2031 (USD Million)
Table 116 Asia-Pacific: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 117 Asia-Pacific: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 118 Asia-Pacific: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 119 Asia-Pacific: RWE Solutions Market, By Application, 2022–2031 (USD Million)
Table 120 Asia-Pacific: RWE Market for Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 121 Asia-Pacific: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 122 Japan: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 123 Japan: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 124 Japan: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 125 Japan: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 126 Japan: RWE Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 127 Japan: RWE Solutions Market by End User, 2022–2031 (USD Million)
Table 128 Diversified Real-world Data Sources in China
Table 129 China: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 130 China: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 131 China: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 132 China: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 133 China: RWE Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 134 China: RWE Solutions Market by End User, 2022–2031 (USD Million)
Table 135 India: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 136 INDIA: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 137 India: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 138 India: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 139 India: RWE Market for Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 140 India: RWE Solutions Market by End User, 2022–2031 (USD Million)
Table 141 South Korea: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 142 South Korea: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 143 South Korea: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 144 South Korea: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 145 South Korea: RWE Market for Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 146 South Korea: RWE Solutions Market by End User, 2022–2031 (USD Million)
Table 147 Australia: RWE Solutions Market, By Component, 2022–2031 (USD Million)
Table 148 Australia: RWE Datasets Market, By Type, 2022–2031 (USD Million)
Table 149 Australia: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 150 Australia: RWE Solutions Market, By Application, 2022–2031 (USD Million)
Table 151 Australia: RWE Market for Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 152 Australia: RWE Solutions Market by End User, 2022–2031 (USD Million)
Table 153 RWE Sources in Taiwan
Table 154 Taiwan: RWE Solutions Market, By Component, 2022–2031 (USD Million)
Table 155 TAIWAN: RWE Datasets Market, By Type, 2022–2031 (USD Million)
Table 156 Taiwan: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 157 Taiwan: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 158 Taiwan: RWE Market for Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 159 Taiwan: RWE Solutions Market by End User, 2022–2031 (USD Million)
Table 160 Currently, the Main Sources for Real-world Data (RWD) in Singapore are:
Table 161 Singapore: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 162 Singapore: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 163 Singapore: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 164 Singapore: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 165 Singapore: RWE Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 166 Singapore: RWE Solutions Market by End User, 2022–2031 (USD Million)
Table 167 Estimated Number of New Cancer Cases from 2022 vs 2025
Table 168 rest OF Asia-Pacific: RWE Solutions Market, By Component, 2022–2031 (USD Million)
Table 169 Rest of Asia-Pacific: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 170 Rest of Asia-Pacific: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 171 Rest of Asia-Pacific: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 172 Rest of Asia-Pacific: RWE Market for Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 173 Rest of Asia-Pacific: RWE Market for Drug Development & Approvals, By End User, 2022–2031 (USD Million)
Table 174 Latin America: RWE Solutions Market, by Country/Region, 2022-2031 (USD Million)
Table 175 Latin America: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 176 Latin America: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 177 Latin America: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 178 Latin America: RWE Solutions Market, by application, 2022–2031 (USD Million)
Table 179 Latin America: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 180 Latin America: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 181 Brazil: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 182 Brazil: RWE Datasets Market, by Type, 2022–2031 (USD Million)
Table 183 Brazil: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 184 Brazil: RWE Solutions Market, by Application, 2022–2031 (USD Million)
Table 185 Brazil: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 186 Brazil: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 187 Mexico: RWE Solutions Market Size, by Component, 2022–2031 (USD Million)
Table 188 Mexico: RWE Datasets Market Size, by Type, 2022–2031 (USD Million)
Table 189 Mexico: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 190 Mexico: RWE Solutions Market, By Application, 2022–2031 (USD Million)
Table 191 Mexico: RWE Solutions Market for Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 192 Mexico: RWE Market for Drug Development & Approvals, By End User, 2022–2031 (USD Million)
Table 193 Rest of Latin America: RWE Solutions Market, By Component, 2022–2031 (USD Million)
Table 194 Rest of Latin America: RWE Datasets Market, By Type, 2022–2031 (USD Million)
Table 195 Rest of Latin America: RWE Disparate Datasets Market, By Type, 2022–2031 (USD Million)
Table 196 Rest of Latin America: RWE Solutions Market, By Application, 2022–2031 (USD Million)
Table 197 Rest of Latin America: RWE Solutions Market for Drug Development & Approvals, by Therapeutic Area, 2022–2031 (USD Million)
Table 198 Rest of Latin America: RWE Solutions Market, by End User, 2022–2031 (USD Million)
Table 199 Middle East & Africa: RWE Solutions Market, by Component, 2022–2031 (USD Million)
Table 200 Middle East & Africa: RWE Datasets Market, By Type, 2022–2031 (USD Million)
Table 201 Middle East & Africa: RWE Disparate Datasets Market, by Type, 2022–2031 (USD Million)
Table 202 Middle East & Africa: RWE Solutions Market Size, By Application, 2022–2031 (USD Million)
Table 203 Middle East & Africa: RWE Solutions Market size For Drug Development & Approvals, By Therapeutic Area, 2022–2031 (USD Million)
Table 204 Middle East & Africa: RWE Solutions Market, By End User, 2022–2031 (USD Million)
Table 205 Recent Developments, By Company, 2021-2024
List of Figures
Figure 1 Research Process
Figure 2 Secondary Sources Referenced For This Study
Figure 3 Primary Research Techniques
Figure 4 Key Executives Interviewed
Figure 5 Breakdown of Primary Interviews (Supply-side & Demand-side)
Figure 6 Market Sizing & Growth Forecast Approach
Figure 7 Global RWE Solutions Market, By Component, 2024 Vs. 2031 (USD Million)
Figure 8 Global RWE Solutions Market, By Application, 2024 Vs. 2031 (USD Million)
Figure 9 Global RWE Solutions Market, By End User, 2024 Vs. 2031 (USD Million)
Figure 10 RWE Solutions Market, By Geography 2024 Vs. 2031 (USD Million)
Figure 11 Impact Analysis of Market Dynamics
Figure 12 Personalized Medicines Approved of Total New Molecular Entities, 2015-2022 (%)
Figure 13 Share of Clinical Trials Conducted Between 2017-2021, By Region (%)
Figure 14 Global Real-World Solutions Market, By Component, 2024 Vs. 2031 (USD Million)
Figure 15 Global Real-World Solutions Market, By Application, 2024 Vs. 2031 (USD Million)
Figure 16 Global Real-World Solutions Market, By End User, 2024 Vs. 2031 (USD Million)
Figure 17 Global RWE Solutions Market, By Country/Region, 2024 Vs. 2031 (USD Million)
Figure 18 North America: RWE Solutions Market Snapshot
Figure 19 U.S: Number of Novel Drug Approvals (2016-2023)
Figure 20 Europe: RWE Solutions Market Snapshot
Figure 21 France: Share of Population Aged 65 and Above, 2017–2023
Figure 22 Spain: Pharmaceutical R&D Expenditure, 2019–2022 (USD Million)
Figure 23 Switzerland: Pharmaceutical R&D Expenditure, 2019–2022 (USD Million)
Figure 24 Pharmaceutical Production in Belgium, 2019–2022 (USD Million)
Figure 25 Asia-Pacific: Real-World Evidence Solutions Market Snapshot
Figure 26 Latin America: RWE Solutions Market Snapshot
Figure 27 Key Growth Strategies Adopted By Leading Players, 2021-2024
Figure 28 Real-World Evidence (RWE) Solutions Market: Competitive Benchmarking, by Component
Figure 29 Real-World Evidence (RWE) Solutions Market: Competitive Benchmarking, by Region
Figure 30 Competitive Dashboard: Real-World Evidence (RWE) Solutions Market
Figure 31 Market Share Analysis: Real-World Evidence (RWE) Solutions Industry, 2023
Figure 32 IQVIA Holdings, Inc.: Financial Overview (2023)
Figure 33 ICON plc: Financial Overview (2023)
Figure 34 Thermo Fisher Scientific, Inc.: Financial Snapshot (2023)
Figure 35 F. Hoffmann-La Roche Ltd.: Financial Snapshot (2023)
Figure 36 Oracle Corporation: Financial Snapshot (2023)
Figure 37 Elevance Health, Inc.: Financial Overview (2023)
Figure 38 Cognizant Technology Solutions Corporation: Financial Snapshot (2023)
Figure 39 Revvity, Inc.: Financial Snapshot (2023)
Figure 40 SAS Institute Inc.: Financial Snapshot (2023)

























Published Date: Jun-2024
Published Date: Jun-2024
Published Date: Dec-2022
Published Date: Oct-2022
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates