Resources
About Us
Pharmacovigilance Market Size, Share, Forecast, & Trends Analysis by Offering (Software [Adverse Event Reporting, Issue Tracking, Drug Safety Audit], Services [Spontaneous Reporting, Case Logging]), Phase, Therapeutic Area, End User – Global Forecast to 2031
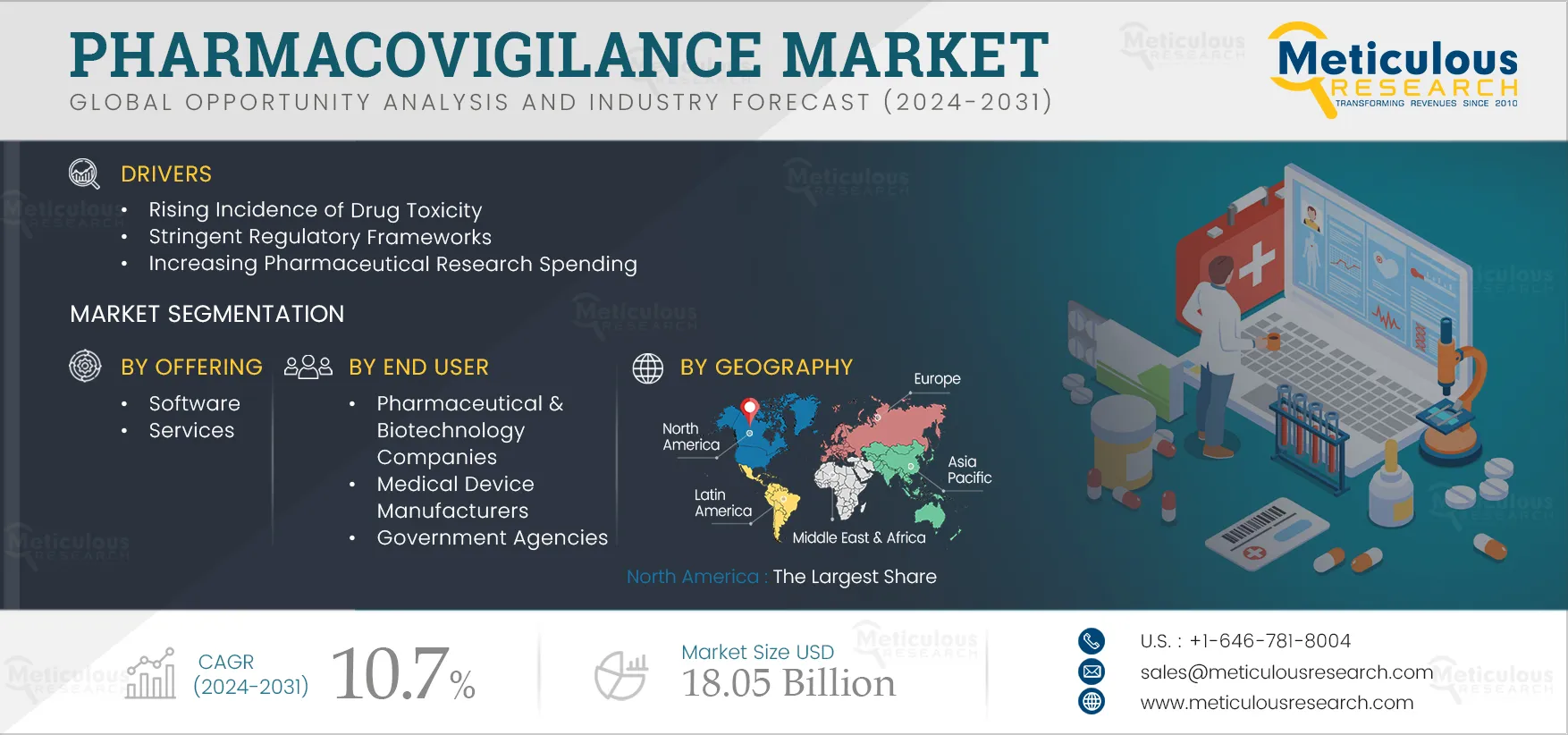
Report ID: MRHC - 1041211 Pages: 400 May-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe growth of this market is primarily driven by the rising incidence of drug toxicity, stringent regulatory frameworks, increasing pharmaceutical research spending, rising need for new drug development, and increasing focus on medical device safety. Furthermore, the introduction of technologically advanced software, growing awareness regarding adverse drug reactions, therapeutic approaches requiring a combination of drugs, and the increasing trend of outsourcing pharmacovigilance are expected to offer growth opportunities for market stakeholders.
Drug toxicity can happen as a result of the over-ingestion of medication, causing too much of the drug to be in the human body. With certain medications, drug toxicity can occur as an adverse drug reaction. ADRs can be serious, affecting patient health or even proving fatal. According to the U.S. FDA, around 6.7% of patients experience serious ADRs, with a 0.3% mortality rate. Hospitalization is also increasing due to ADRs. According to the CDC, ADRs cause around 1.3 million emergency department visits each year in the U.S., of which around 35,000 patients are hospitalized immediately after their emergency department visit.
The incidence of ADRs varies and depends on factors such as age, sex, ethnicity, existing disorders, genetic and environmental factors, administration route, treatment duration, dosage, and drug type. Moreover, increased age and polypharmacy also lead to ADRs. Pharmacovigilance is used to detect problems in medicine usage, reduce risks, and communicate observations in a disciplined way.
Click here to: Get a Free Sample Copy of this Report
Medical device companies, pharmaceutical companies, and Contract Research Organizations (CROs) are constantly under pressure to meet time-critical regulatory requirements using limited resources. Companies should manage and identify drug safety events before they become fatal for patients. Companies also need to maintain rigorous compliance with evolving regulations, necessitating the use of pharmacovigilance software and services.
Pharmacovigilance gathers and analyzes information about regulatory frameworks and policies, helping these companies make informed decisions about drug development and testing. Pharmacovigilance also helps in assessing the impact of regulatory changes on a company’s product portfolio. Thus, frequently changing regulatory frameworks are increasing the demand for pharmacovigilance services.
Integration of AI into pharmacovigilance is important in task automation, efficient and effective adverse event detection & analysis, improvements in data processing, and optimization of pharmacovigilance processes. AI's role in pharmacovigilance includes improved clinical trial prioritization, faster adverse event detection, and accurate dosing recommendations. By analyzing large datasets and automating processes, AI enhances drug safety, monitoring, and patient care in the pharmaceutical industry.
AI is used to automate the identification of adverse event detection in large datasets. AI can extract information directly from sources like medical literature, electronic health records, and social media to detect and categorize adverse events. AI also helps automate the case report generation process by obtaining appropriate information from unstructured data sources, thus reducing the time & effort required for manual data entry. Machine Learning (ML) algorithms can predict possible drug-drug interactions by examining patient data and detecting drug combinations that may pose a risk.
The pharmaceutical & life sciences sector is currently shifting toward cloud-based drug safety platforms for easy data processing, collection, and analysis, and adverse event reporting. The use of cloud-based tools & technologies has accelerated digital transformation in pharmacovigilance and drug safety. Cloud computing helps in digital transformation by offering a platform that is much more efficient, scalable, secure, and cost-effective, manages high volumes of data, and automates drug safety processes.
One of the main benefits offered by cloud-based solutions is the ease of upgrading systems. As the regulations for drug safety continuously change or new technological innovations emerge, cloud-based systems analyzing data regarding drug adverse events are automatically upgraded to support the new requirements. This can be useful for pharmaceutical companies as on-premises applications are considered extremely expensive and sometimes time-consuming.
The trend of outsourcing pharmacovigilance is increasing among pharmaceutical & biotechnology companies and medical device manufacturers. Instead of setting up and maintaining an in-house pharmacovigilance department, pharmaceutical companies are partnering with external service providers for resource optimization and management. Outsourcing allows partner companies to optimize resource allocation according to project demand. Outsourcing also offers an advantage in terms of time and customization of services. Benefits such as increased business capacity & model flexibility, on-demand access to specialized expertise, and multidisciplinary as well as intellectual property knowledge are driving the demand for outsourced pharmacovigilance services. With the increasing number of pharmaceutical companies, the demand for pharmacovigilance outsourcing is also increasing. According to IQVIA (U.S.), 67% of pharmaceutical and biotechnology companies outsource their pharmacovigilance operations, and 54% outsource their regulatory activities.
Based on offering, the pharmacovigilance market is segmented into software and services. In 2024, the services segment is expected to account for the larger share of 97.6% of the pharmacovigilance market. This segment is also projected to record the higher CAGR of 10.8% during the forecast period 2024–2031. The increasing number of molecules in clinical development and the growing importance of drug safety are driving the outsourcing of pharmacovigilance services, contributing to the segment’s large share. In 2023, the U.S. FDA approved 55 drugs in comparison to 37 drugs in 2022 (source: U.S. FDA). The rising number of drug approvals drives the demand for pharmacovigilance services, supporting the growth of this segment.
Based on drug development phase, the pharmacovigilance market is segmented into Phase IV, Phase III, Phase II, Phase I, and preclinical studies. In 2024, the phase IV segment is expected to account for the largest share of 72.5% of the pharmacovigilance market. Phase IV clinical trials involve larger patient populations, increasing the likelihood of rare adverse events. Phase IV clinical trials monitor the effectiveness and safety of drugs. The demand for pharmacovigilance services & software is high as the volume of data generated from real-world evidence is significant. During Phase IV, undetected or unsuspected ADRs can be detected due to testing being conducted on a large number of patients and over a longer observation period. Thus, the high complexity of Phase IV is increasing the adoption of pharmacovigilance services for the efficient management of clinical trials.
Based on therapeutic area, the pharmacovigilance market is segmented into oncology, cardiology/vascular diseases, infectious diseases, immunology, neurology, and other therapeutic areas. In 2024, the oncology segment is expected to account for the largest share of the pharmacovigilance market. The segment’s large share is attributed to the availability of funding for oncology, life science & healthcare research, the increasing demand for personalized therapies, and high cancer incidence necessitating newer therapies to be launched in the market.
Biopharmaceutical companies’ growing emphasis on research activities and rising government support for the development of cancer treatment are supporting the outsourcing of pharmacovigilance services. For instance, in February 2024, BioNTech SE (Germany) invested USD 200 million in Autolus Therapeutics (U.K.) and collaborated with the company to support the cancer therapy pipeline. Pharmacovigilance solutions monitor patient safety during treatment for the detection of long-term complications and adverse events, contributing to oncology drug safety.
Based on end user, the pharmacovigilance market is segmented into pharmaceutical & biotechnology companies, medical device manufacturers, government agencies, and other end users. In 2024, the pharmaceutical & biotechnology companies segment is expected to account for the largest share of the pharmacovigilance market. The segment’s large share is attributed to pharmaceutical & biotechnology companies’ high investments in research & development. For instance, global pharmaceutical R&D expenditure is expected to reach USD 213 billion in 2026 from USD 179 billion in 2020 (Source: International Federation of Pharmaceutical Manufacturers & Associations - IFPMA). This growing focus on R&D is supporting the development of newer therapies, driving the need for pharmacovigilance services.
In 2024, North America is expected to account for the largest share of 44.3% of the pharmacovigilance market. North America’s large share is attributed to the presence of key market players, advanced infrastructure and resources supporting research & development, the large number of clinical trials and drug approvals, and increased reporting of adverse drug events in the region. The number of drugs in the pipeline is also increasing in North America. For instance, AbbVie Inc. (U.S.) has more than 90 indications, compounds, or devices in development, individually or in collaboration. According to Pharmaceutical Research and Manufacturers of America (U.S.), 1,600 vaccines and treatments for cancer are under development. Thus, the region’s significant drug pipeline is increasing the need for pharmacovigilance solutions for monitoring drug safety.
However, Asia-Pacific is slated to register the highest growth rate of 12.3% during the forecast period. The countries in Asia-Pacific, including China, India, and South Korea, are expected to hold significant growth opportunities for the vendors in this market. Growing cancer incidence, rising awareness about ADRs, and increasing funding and supportive initiatives for drug discovery are driving the growth of this regional market. For instance, in July 2022, the Council of Scientific and Industrial Research (CSIR) (India) invested USD 6.73 million in R&D activities for drug discovery and development. Additionally, the increasing number of clinical trials in the region is driving the growth of the pharmacovigilance market. According to the WHO, the number of clinical trials in Southeast Asia increased to 11,030 in 2022 from 9,175 in 2020.
The report includes a competitive landscape based on an extensive assessment of the product offerings and geographic presence of leading market players and the key growth strategies adopted by them over the past few years. The key players operating in the pharmacovigilance market are IQVIA (U.S.), Cognizant Technology Solutions Corporation (U.S.), Linical Co., Ltd (U.S.), International Business Machines Corporation (U.S.), Laboratory Corporation of America Holdings (U.S.), ICON plc (Ireland), Parexel International (U.S.), Wipro Limited (India), Sanofi S.A. (France), Pharmaceutical Product Development Inc. (a subsidiary of Thermo Fisher Scientific Inc.) (U.S.), Capgemini SE (France), Syneos Health (U.S.), ArisGlobal (U.S.), Ennov (France), EXTEDO GmbH (Germany), Oracle Corporation (U.S.), Sparta Systems Inc. (U.S.), United BioSource, LLC (UBC) (U.S.), and AB Cube S.A.S. (France).
In December 2023, Pharmaceutical Product Development, Inc., a subsidiary of Thermo Fisher Scientific Inc. (U.S.), launched CorEvidence, a patented cloud-based data lake platform for the optimization of pharmacovigilance case processing and safety data management processes.
In March 2023, ArisGlobal (U.S.) launched Intelligent Content Management for Safety. The solution can organize and store extensive documentation related to safety activities. It builds on LifeSphere’s Clinical counterpart, which supports the electronic Trial Master File (eTMF) solution.
|
Particulars |
Details |
|
Number of Pages |
400 |
|
Format |
|
|
Forecast Period |
2024-2031 |
|
Base Year |
2023 |
|
CAGR |
10.7% |
|
Estimated Market Size (Value) |
$18.05 Billion by 2031 |
|
Segments Covered |
By Offering
By Drug Development Phase
By Therapeutic Area
By End User
|
|
Countries Covered |
North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Belgium, Netherlands, Denmark, Sweden, and Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Australia, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East & Africa |
|
Key Companies Profiled |
IQVIA (U.S.), Cognizant Technology Solutions Corporation (U.S.), Linical Co., Ltd (U.S.), International Business Machines Corporation (U.S.), Laboratory Corporation of America Holdings (U.S.), ICON plc (Ireland), Parexel International (U.S.), Wipro Limited (India), Sanofi S.A. (France), Pharmaceutical Product Development Inc. (a subsidiary of Thermo Fisher Scientific Inc.) (U.S.), Capgemini SE (France), Syneos Health (U.S.), ArisGlobal (U.S.), Ennov (France), EXTEDO GmbH (Germany), Oracle Corporation (U.S.), Sparta Systems Inc. (U.S.), United BioSource, LLC (UBC) (U.S.), and AB Cube S.A.S. (France) |
The pharmacovigilance market report covers the qualitative and quantitative assessment of the pharmacovigilance market. This report provides market sizes and forecasts for pharmacovigilance market segments and subsegments by offering, drug development phase, therapeutic area, and end user at the regional and country levels. The report also provides insights on factors impacting market growth, along with a regulatory analysis and Porter’s Five Forces analysis for the pharmacovigilance market.
The pharmacovigilance market is projected to reach $18.05 billion by 2031 at a CAGR of 10.7% from 2024 to 2031.
Based on offering, in 2024, the services segment is expected to account for the larger share of 97.6% of the pharmacovigilance market. The increasing number of molecules in clinical development and the growing importance of drug safety are driving the outsourcing of pharmacovigilance services, contributing to the segment’s large share.
Based on therapeutic area, in 2024, the oncology segment is expected to account for the largest share of the pharmacovigilance market. According to GLOBOCAN, global cancer prevalence is expected to reach 32.64 million in 2045 from 19.98 million in 2022. The rising incidence of cancer is increasing the demand for newer therapies and drugs, increasing the adoption of pharmacovigilance solutions.
The growth of the pharmacovigilance market is driven by the rising incidence of drug toxicity, stringent regulatory frameworks, increasing pharmaceutical research spending, the rising need for new drugs, increasing public awareness regarding drug safety, and the rising focus on medical device pharmacovigilance. Furthermore, the introduction of technologically advanced software, growing awareness regarding adverse drug reactions, growing cases of diseases requiring a combination of drugs, and the increasing outsourcing of pharmacovigilance services are expected to generate growth opportunities for the stakeholders in this market.
The key players operating in the pharmacovigilance market are IQVIA (U.S.), Cognizant Technology Solutions Corporation (U.S.), Linical Co., Ltd (U.S.), International Business Machines Corporation (U.S.), Laboratory Corporation of America Holdings (U.S.), ICON plc (Ireland), Parexel International (U.S.), Wipro Limited (India), Sanofi S.A. (France), Pharmaceutical Product Development Inc. (a subsidiary of Thermo Fisher Scientific Inc.) (U.S.), Capgemini SE (France), Syneos Health (U.S.), ArisGlobal (U.S.), Ennov (France), EXTEDO GmbH (Germany), Oracle Corporation (U.S.), Sparta Systems Inc. (U.S.), United BioSource, LLC (UBC) (U.S.), and AB Cube S.A.S. (France).
Asia-Pacific is slated to register the highest growth rate during the forecast period. The high growth of this regional market is attributed to increasing research on vaccines, policies supporting the adoption of pharmacovigilance, increasing pharmaceutical R&D expenditures, a growing focus on personalized medicine, and advancements in the biotechnology sector in Asia-Pacific.
1. Introduction
1.1. Market Definition & Scope
1.2. Market Ecosystem
1.3. Currency & Limitations
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Approach
2.2. Data Collection & Validation Process
2.2.1. Secondary Research
2.2.2. Primary Research/Interviews with Key Opinion Leaders from the Industry
2.3. Market Sizing & Forecasting
2.3.1. Market Size Estimation Approach
2.3.2. Growth Forecast Approach
2.3.3. Market Share Analysis
2.4. Assumptions for the Study
3. Executive Summary
4. Market Insights
4.1. Overview
4.2. Drivers
4.2.1. Rising Incidence of Drug Toxicity
4.2.2. Stringent Regulatory Frameworks
4.2.3. Increasing Pharmaceutical Research Spending
4.2.4. Rising Need for the Development of New Drugs
4.2.5. Growing Focus on Medical Device Safety
4.3. Restraints
4.3.1. High Costs of Pharmacovigilance Services
4.4. Opportunities
4.4.1. Introduction of Technologically Advanced Software
4.4.2 Growing Awareness Regarding the Reporting of Adverse Drug Reactions
4.4.3. Therapeutic Approaches Requiring Drug Combinations
4.4.4. Increasing Outsourcing of Pharmacovigilance Services
4.5. Challenges
4.5.1. Data Security Risks
4.5.2. Shortage of Skilled Pharmacovigilance Professionals
4.6. Factor Analysis
4.7. Trends
4.8. Regulatory Analysis
4.9. Porter’s Five Force Analysis
5. Pharmacovigilance Market Assessment—by Offering
5.1. Overview
5.2. Software
5.2.1. Software, by Deployment Mode
5.2.1.1. On-premise
5.2.1.2. Cloud-based
5.2.2. Software, by Functionality
5.2.2.1. Issue Tracking Software
5.2.2.2. Adverse Event Reporting Software
5.2.2.3. Fully Integrated Software
5.2.2.4. Drug Safety Audit Software
5.2.2.5. Other Functionalities
5.3. Services
5.3.1. Services, by Service Provider
5.3.1.1. In-house
5.3.1.2. Contract Outsourcing
5.3.2. Services, by Type
5.3.2.1. Spontaneous Reporting
5.3.2.2. Intensified ADR Reporting
5.3.2.3. Targeted Spontaneous Reporting
5.3.2.4. Cohort Event Monitoring
5.3.2.5. EHR Mining
5.3.2.6. Other Services
5.3.3. Services, by process
5.3.3.1. Core Services
5.3.3.2. Consulting Services
5.3.4. Services, by Process Outflow
5.3.4.1. Case Data Management
5.3.4.1.1. Case Logging
5.3.4.1.2. Case Data Analysis
5.3.4.1.3. Medical Review & Reporting
5.3.4.2. Signal Detection
5.3.4.2.1. Adverse Event Logging
5.3.4.2.2. Adverse Event Analysis
5.3.4.2.3. Adverse Event Review & Reporting
5.3.4.3. Risk Management Systems
5.3.4.3.1. Risk Evaluation Systems
5.3.4.3.2. Risk Mitigation Systems
6. Pharmacovigilance Market Assessment—by Drug Development Phase
6.1. Overview
6.2. Phase IV
6.3. Phase III
6.4. Phase II
6.5. Phase I
6.6. Preclinical Studies
7. Pharmacovigilance Market Assessment—by Therapeutic Area
7.1. Oncology
7.2. Cardiology/Vascular Diseases
7.3. Infectious Diseases
7.4. Immunology
7.5. Neurology
7.6. Other Therapeutic Areas
8. Pharmacovigilance Market Assessment—by End User
8.1. Overview
8.2. Pharmaceutical & Biotechnology Companies
8.3. Medical Device Manufacturers
8.4. Government Agencies
8.5. Other End Users
9. Pharmacovigilance Market Assessment—by Geography
9.1. Overview
9.2. North America
9.2.1. U.S.
9.2.2. Canada
9.3. Europe
9.3.1. Germany
9.3.2. France
9.3.3. U.K.
9.3.4. Italy
9.3.5. Spain
9.3.6. Belgium
9.3.7. Netherlands
9.3.8. Denmark
9.3.9. Sweden
9.3.10. Rest of Europe (RoE)
9.4. Asia-Pacific
9.4.1. Japan
9.4.2. China
9.4.3. India
9.4.4. South Korea
9.4.5. Australia
9.4.6. Rest of Asia-Pacific (RoAPAC)
9.5. Latin America
9.5.1. Brazil
9.5.2. Mexico
9.5.3. Rest of Latin America (RoLATAM)
9.6. Middle East & Africa
10. Competition Analysis
10.1. Overview
10.2. Key Growth Strategies
10.3. Competitive Benchmarking
10.4. Competitive Dashboard
10.4.1. Industry Leaders
10.4.2. Market Differentiators
10.4.3. Vanguards
10.4.4. Emerging Companies
10.5. Market Share Analysis/Market Rankings of Key Players (2023)
11. Company Profiles (Company Overview, Financial Overview, Product Portfolio, Strategic Developments, and *SWOT Analysis)
11.1. IQVIA
11.2. Cognizant Technology Solutions Corporation
11.3. Linical Co., Ltd
11.4. International Business Machines Corporation
11.5. Laboratory Corporation of America Holdings
11.6. ICON plc
11.7. Parexel International
11.8. Wipro Limited
11.9. Sanofi S.A.
11.10 Pharmaceutical Product Development, Inc. (A Subsidiary of Thermo Fisher Scientific Inc.)
11.11. Capgemini SE
11.12. Syneos Health
11.13. ArisGlobal
11.14. Ennov
11.15. EXTEDO GmbH
11.16. Oracle Corporation
11.17. Sparta Systems Inc
11.18. United BioSource, LLC (UBC)
11.19. AB Cube S.A.S.
(Note: SWOT analysis will be provided for the top 5 companies.)
12. Appendix
12.1. Available Customization
12.2. Related Reports
List of Tables
Table 1 Global Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 2 Global Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 3 Global Pharmacovigilance Software Market, by Country/Region, 2022–2031 (USD Million)
Table 4 Global On-premise Pharmacovigilance Software Market, by Country/Region, 2022–2031 (USD Million)
Table 5 Global Cloud-based Pharmacovigilance Software Market, by Country/Region, 2022–2031 (USD Million)
Table 6 Global Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 7 Global Pharmacovigilance Issue Tracking Software Market, by Country/Region, 2022–2031 (USD Million)
Table 8 Global Pharmacovigilance Adverse Event Reporting Software Market, by Country/Region, 2022–2031 (USD Million)
Table 9 Global Pharmacovigilance Fully Integrated Software Market, by Country/Region, 2022–2031 (USD Million)
Table 10 Global Pharmacovigilance Drug Safety Audit Software Market, by Country/Region, 2022–2031 (USD Million)
Table 11 Global Pharmacovigilance Software Market for Other Functionalities, by Country/Region, 2022–2031 (USD Million)
Table 12 Global Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 13 Global Pharmacovigilance Services Market, by Country/Region, 2022–2031 (USD Million)
Table 14 Global In-house Pharmacovigilance Services Market, by Country/Region, 2022–2031 (USD Million)
Table 15 Global Contract Outsourcing Pharmacovigilance Services Market, by Country/Region, 2022–2031 (USD Million)
Table 16 Global Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 17 Global Pharmacovigilance Spontaneous Reporting Services Market, by Country/Region, 2022–2031 (USD Million)
Table 18 Global Pharmacovigilance Intensified ADR Reporting Services Market, by Country/Region, 2022–2031 (USD Million)
Table 19 Global Pharmacovigilance Targeted Spontaneous Reporting Services Market, by Country/Region, 2022–2031 (USD Million)
Table 20 Global Pharmacovigilance Cohort Event Monitoring Services Market, by Country/Region, 2022–2031 (USD Million)
Table 21 Global Pharmacovigilance EHR Mining Services Market, by Country/Region, 2022–2031 (USD Million)
Table 22 Global Other Pharmacovigilance Services Market, by Country/Region, 2022–2031 (USD Million)
Table 23 Global Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 24 Global Pharmacovigilance Core Services Market, by Country/Region, 2022–2031 (USD Million)
Table 25 Global Pharmacovigilance Consulting Services Market, by Country/Region, 2022–2031 (USD Million)
Table 26 Global Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 27 Global Pharmacovigilance Case Data Management Services Market, by Type, 2022–2031 (USD Million)
Table 28 Global Pharmacovigilance Case Data Management Services Market, by Country/Region, 2022–2031 (USD Million)
Table 29 Global Pharmacovigilance Case Logging Services Market, by Country/Region, 2022–2031 (USD Million)
Table 30 Global Pharmacovigilance Case Data Analysis Services Market, by Country/Region, 2022–2031 (USD Million)
Table 31 Global Pharmacovigilance Medical Review & Reporting Services Market, by Country/Region, 2022–2031 (USD Million)
Table 32 Global Pharmacovigilance Signal Detection Services Market, by Type, 2022–2031 (USD Million)
Table 33 Global Pharmacovigilance Signal Detection Services Market, by Country/Region, 2022–2031 (USD Million)
Table 34 Global Pharmacovigilance Adverse Event Logging Services Market, by Country/Region, 2022–2031 (USD Million)
Table 35 Global Pharmacovigilance Adverse Event Analysis Services Market, by Country/Region, 2022–2031 (USD Million)
Table 36 Global Pharmacovigilance Adverse Event Review & Reporting Services Market, by Country/Region, 2022–2031 (USD Million)
Table 37 Global Pharmacovigilance Risk Management System Services Market, by Type, 2022–2031 (USD Million)
Table 38 Global Pharmacovigilance Risk Management System Services Market, by Country/Region, 2022–2031 (USD Million)
Table 39 Global Pharmacovigilance Risk Evaluation System Services Market, by Country/Region, 2022–2031 (USD Million)
Table 40 Global Pharmacovigilance Risk Mitigation System Services Market, by Country/Region, 2022–2031 (USD Million)
Table 41 Global Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 42 Global Pharmacovigilance Market for Phase IV, by Country/Region, 2022–2031 (USD Million)
Table 43 Global Pharmacovigilance Market for Phase III, by Country/Region, 2022–2031 (USD Million)
Table 44 Global Pharmacovigilance Market for Phase II, by Country/Region, 2022–2031 (USD Million)
Table 45 Global Pharmacovigilance Market for Phase I, by Country/Region, 2022–2031 (USD Million)
Table 46 Global Pharmacovigilance Market for Preclinical Studies, by Country/Region, 2022–2031 (USD Million)
Table 47 Global Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 48 Global Pharmacovigilance Market for Oncology, by Country/Region, 2022–2031 (USD Million)
Table 49 Global Pharmacovigilance Market for Cardiology/Vascular Diseases, by Country/Region, 2022–2031 (USD Million)
Table 50 Global Pharmacovigilance Market for Infectious Diseases, by Country/Region, 2022–2031 (USD Million)
Table 51 Global Pharmacovigilance Market for Immunology, by Country/Region, 2022–2031 (USD Million)
Table 52 Global Pharmacovigilance Market for Neurology, by Country/Region, 2022–2031 (USD Million)
Table 53 Global Pharmacovigilance Market for Other Therapeutic Areas, by Country/Region, 2022–2031 (USD Million)
Table 54 Global Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 55 Global Pharmacovigilance Market for Pharmaceutical & Biotechnology Companies, by Country/Region, 2022–2031 (USD Million)
Table 56 Global Pharmacovigilance Market for Medical Device Manufacturers, by Country/Region, 2022–2031 (USD Million)
Table 57 Global Pharmacovigilance Market for Government Agencies, by Country/Region, 2022–2031 (USD Million)
Table 58 Global Pharmacovigilance Market for Other End Users, by Country/Region, 2022–2031 (USD Million)
Table 59 Global Pharmacovigilance Market, by Country/Region, 2022–2031 (USD Million)
Table 60 North America: Pharmacovigilance Market, by Country, 2022–2031 (USD Million)
Table 61 North America: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 62 North America: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 63 North America: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 64 North America: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 65 North America: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 66 North America: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 67 North America: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 68 North America: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 69 North America: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 70 North America: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 71 U.S.: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 72 U.S.: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 73 U.S.: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 74 U.S.: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 75 U.S.: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 76 U.S.: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 77 U.S.: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 78 U.S.: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 79 U.S.: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 80 U.S.: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 81 Canada: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 82 Canada: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 83 Canada: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 84 Canada: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 85 Canada: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 86 Canada: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 87 Canada: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 88 Canada: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 89 Canada: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 90 Canada: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 91 Europe: Pharmacovigilance Market, by Country/Region, 2022–2031 (USD Million)
Table 92 Europe: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 93 Europe: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 94 Europe: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 95 Europe: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 96 Europe: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 97 Europe: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 98 Europe: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 99 Europe: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 100 Europe: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 101 Europe: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 102 Germany: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 103 Germany: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 104 Germany: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 105 Germany: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 106 Germany: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 107 Germany: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 108 Germany: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 109 Germany: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 110 Germany: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 111 Germany: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 112 France: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 113 France: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 114 France: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 115 France: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 116 France: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 117 France: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 118 France: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 119 France: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 120 France: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 121 France: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 122 U.K.: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 123 U.K.: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 124 U.K.: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 125 U.K.: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 126 U.K.: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 127 U.K.: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 128 U.K.: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 129 U.K.: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 130 U.K.: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 131 U.K.: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 132 Italy: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 133 Italy: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 134 Italy: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 135 Italy: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 136 Italy: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 137 Italy: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 138 Italy: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 139 Italy: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 140 Italy: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 141 Italy: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 142 Spain: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 143 Spain: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 144 Spain: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 145 Spain: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 146 Spain: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 147 Spain: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 148 Spain: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 149 Spain: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 150 Spain: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 151 Spain: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 152 Belgium: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 153 Belgium: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 154 Belgium: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 155 Belgium: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 156 Belgium: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 157 Belgium: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 158 Belgium: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 159 Belgium: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 160 Belgium: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 161 Belgium: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 162 Netherlands: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 163 Netherlands: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 164 Netherlands: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 165 Netherlands: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 166 Netherlands: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 167 Netherlands: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 168 Netherlands: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 169 Netherlands: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 170 Netherlands: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 171 Netherlands: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 172 Denmark: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 173 Denmark: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 174 Denmark: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 175 Denmark: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 176 Denmark: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 177 Denmark: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 178 Denmark: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 179 Denmark: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 180 Denmark: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 181 Denmark: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 182 Sweden: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 183 Sweden: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 184 Sweden: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 185 Sweden: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 186 Sweden: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 187 Sweden: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 188 Sweden: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 189 Sweden: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 190 Sweden: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 191 Sweden: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 192 Rest of Europe: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 193 Rest of Europe: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 194 Rest of Europe: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 195 Rest of Europe: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 196 Rest of Europe: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 197 Rest of Europe: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 198 Rest of Europe: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 199 Rest of Europe: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 200 Rest of Europe: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 201 Rest of Europe: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 202 Asia-Pacific: Pharmacovigilance Market, by Country/Region, 2022–2031 (USD Million)
Table 203 Asia-Pacific: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 204 Asia-Pacific: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 205 Asia-Pacific: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 206 Asia-Pacific: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 207 Asia-Pacific: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 208 Asia-Pacific: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 209 Asia-Pacific: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 210 Asia-Pacific: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 211 Asia-Pacific: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 212 Asia-Pacific: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 213 China: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 214 China: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 215 China: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 216 China: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 217 China: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 218 China: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 219 China: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 220 China: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 221 China: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 222 China: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 223 Japan: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 224 Japan: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 225 Japan: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 226 Japan: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 227 Japan: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 228 Japan: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 229 Japan: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 230 Japan: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 231 Japan: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 232 Japan: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 233 India: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 234 India: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 235 India: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 236 India: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 237 India: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 238 India: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 239 India: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 240 India: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 241 India: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 242 India: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 243 South Korea: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 244 South Korea: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 245 South Korea: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 246 South Korea: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 247 South Korea: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 248 South Korea: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 249 South Korea: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 250 South Korea: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 251 South Korea: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 252 South Korea: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 253 Australia: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 254 Australia: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 255 Australia: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 256 Australia: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 257 Australia: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 258 Australia: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 259 Australia: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 260 Australia: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 261 Australia: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 262 Australia: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 263 Rest of Asia-Pacific: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 264 Rest of Asia-Pacific: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 265 Rest of Asia-Pacific: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 266 Rest of Asia-Pacific: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 267 Rest of Asia-Pacific: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 268 Rest of Asia-Pacific: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 269 Rest of Asia-Pacific: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 270 Rest of Asia-Pacific: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 271 Rest of Asia-Pacific: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 272 Rest of Asia-Pacific: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 273 Latin America: Pharmacovigilance Market, by Country/Region, 2022–2031 (USD Million)
Table 274 Latin America: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 275 Latin America: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 276 Latin America: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 277 Latin America: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 278 Latin America: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 279 Latin America: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 280 Latin America: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 281 Latin America: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 282 Latin America: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 283 Latin America: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 284 Brazil: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 285 Brazil: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 286 Brazil: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 287 Brazil: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 288 Brazil: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 289 Brazil: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 290 Brazil: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 291 Brazil: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 292 Brazil: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 293 Brazil: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 294 Mexico: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 295 Mexico: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 296 Mexico: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 297 Mexico: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 298 Mexico: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 299 Mexico: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 300 Mexico: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 301 Mexico: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 302 Mexico: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 303 Mexico: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 304 Rest of Latin America: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 305 Rest of Latin America: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 306 Rest of Latin America: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 307 Rest of Latin America: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 308 Rest of Latin America: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 309 Rest of Latin America: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 310 Rest of Latin America: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 311 Rest of Latin America: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 312 Rest of Latin America: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 313 Rest of Latin America: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 314 Middle East & Africa: Pharmacovigilance Market, by Offering, 2022–2031 (USD Million)
Table 315 Middle East & Africa: Pharmacovigilance Software Market, by Deployment Mode, 2022–2031 (USD Million)
Table 316 Middle East & Africa: Pharmacovigilance Software Market, by Functionality, 2022–2031 (USD Million)
Table 317 Middle East & Africa: Pharmacovigilance Services Market, by Service Provider, 2022–2031 (USD Million)
Table 318 Middle East & Africa: Pharmacovigilance Services Market, by Type, 2022–2031 (USD Million)
Table 319 Middle East & Africa: Pharmacovigilance Services Market, by Process, 2022–2031 (USD Million)
Table 320 Middle East & Africa: Pharmacovigilance Services Market, by Process Outflow, 2022–2031 (USD Million)
Table 321 Middle East & Africa: Pharmacovigilance Market, by Drug Development Phase, 2022–2031 (USD Million)
Table 322 Middle East & Africa: Pharmacovigilance Market, by Therapeutic Area, 2022–2031 (USD Million)
Table 323 Middle East & Africa: Pharmacovigilance Market, by End User, 2022–2031 (USD Million)
Table 324 Recent Developments, by Company, 2020–2024
List of Figures
Figure 1 Research Process
Figure 2 Key Secondary Sources
Figure 3 Primary Research Techniques
Figure 4 Key Executives Interviewed
Figure 5 Breakdown of Primary Interviews (Supply-side & Demand-side)
Figure 6 Market Size Estimation
Figure 7 Global Pharmacovigilance Market, by Offering, 2024 Vs. 2031 (USD Million)
Figure 8 Global Pharmacovigilance Market, by Drug Development Phase, 2024 Vs. 2031 (USD Million)
Figure 9 Global Pharmacovigilance Market, by Therapeutic Area, 2024 Vs. 2031 (USD Million)
Figure 10 Global Pharmacovigilance Market, by End User, 2024 Vs. 2031 (USD Million)
Figure 11 Global Pharmacovigilance Market, by Geography
Figure 12 Global Pharmacovigilance Market, by Offering, 2024 Vs. 2031 (USD Million)
Figure 13 Global Pharmacovigilance Market, by Drug Development Phase, 2024 Vs. 2031 (USD Million)
Figure 14 Global Pharmacovigilance Market, by Therapeutic Area, 2024 Vs. 2031 (USD Million)
Figure 15 Global Pharmacovigilance Market, by End User, 2024 Vs. 2031 (USD Million)
Figure 16 Global Pharmacovigilance Market, by Geography, 2024 Vs. 2031 (USD Million)
Figure 17 North America: Pharmacovigilance Market Snapshot
Figure 18 Europe: Pharmacovigilance Market Snapshot
Figure 19 Asia-Pacific: Pharmacovigilance Market Snapshot
Figure 20 Latin America: Pharmacovigilance Market Snapshot
Figure 21 Key Growth Strategies Adopted by Leading Players, 2020–2024
Figure 22 Global Pharmacovigilance Market: Competitive Benchmarking, by Offering
Figure 23 Global Pharmacovigilance Market: Competitive Benchmarking, by Geography
Figure 24 Global Pharmacovigilance Market: Competitive Dashboard
Figure 25 Global Pharmacovigilance Market: Market Share Analysis/Market Rankings (2023)
Figure 26 IQVIA: Financial Snapshot (2023)
Figure 27 Cognizant Technology Solutions Corporation: Financial Snapshot (2023)
Figure 28 Linical Co., Ltd.: Financial Snapshot (2023)
Figure 29 International Business Machines Corporation: Financial Snapshot (2023)
Figure 30 Laboratory Corporation of America Holdings: Financial Snapshot (2023)
Figure 31 ICON plc: Financial Snapshot (2023)
Figure 32 Wipro Limited: Financial Snapshot (2023)
Figure 33 Sanofi S.A.: Financial Snapshot (2023)
Figure 34 Pharmaceutical Product Development Inc.: Financial Snapshot (2023)
Figure 35 Capgemini SE: Financial Snapshot (2023)
Figure 36 Syneos Health: Financial Snapshot (2023)
Figure 37 Oracle Corporation: Financial Snapshot (2023)

























Published Date: Jun-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates