Resources
About Us
North America Pharmaceutical Contract Development and Manufacturing Market by Service {Manufacturing [API, FDF (Parenteral, Tablet, Capsule, Oral Liquid)], Drug Development, Biologics Manufacturing, Packaging}, End User [Large Pharma] - Forecast to 2032
Report ID: MRHC - 1041066 Pages: 150 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe North America Pharmaceutical Contract Development and Manufacturing Market is expected to reach $105.42 billion by 2032 at a CAGR of 7.1% from 2025 to 2032. Pharmaceutical contract development and manufacturing organizations (CDMOs) partner with pharmaceutical companies and offer contract-based manufacturing and drug development services. CDMOs handle everything from pre-formulation and formulation development to clinical trials and manufacturing. Pharmaceutical companies opt for CDMOs because of the benefits such as reduced labor, cost, high product quality, faster time, and resource savings.
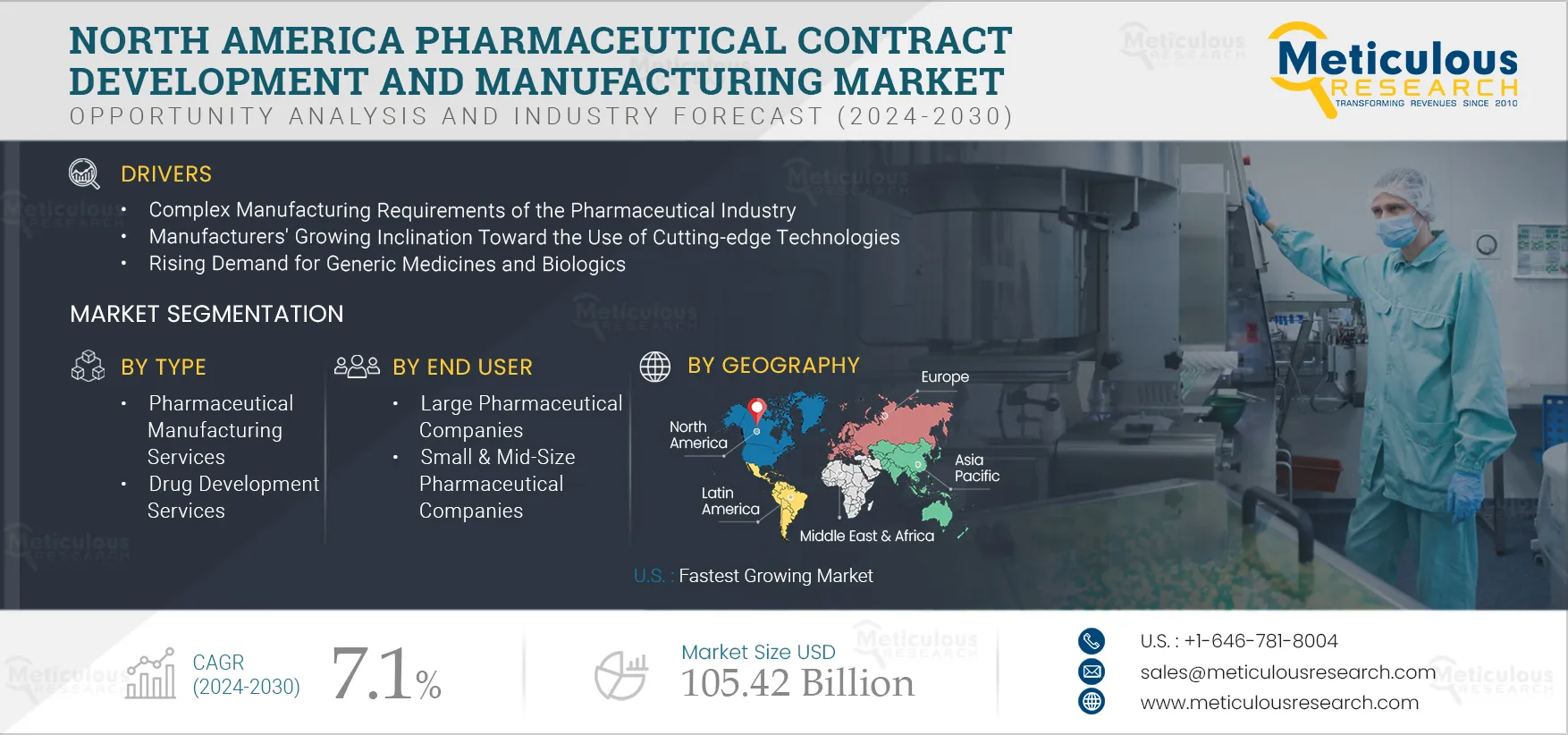
The growth of the North America pharmaceutical contract development and manufacturing market is attributed to the complex manufacturing requirements of the pharmaceutical industry, manufacturers’ growing inclination towards the use of cutting-edge technologies, patent expiration, increasing investments in pharmaceutical R&D, and the rising demand for generic medicines & biologics. However, the lack of skilled professionals is challenging the growth of this market. In addition, growing consolidation among CDMO market players and virtual business models are the industry trends in the market.
Next-generation medicines, such as cell and gene therapy, provide significant opportunities for growth in CRO and CDMO marketplaces. Gene therapy alters the genes to cure disease or improve the body's ability to fight disease. Also, cell therapy is a therapy in which viable cells are injected, grafted, or implanted into a patient to effectuate a medicinal effect. Potential applications of cell therapies include treating cancers, autoimmune diseases, urinary problems, and infectious diseases, rebuilding damaged cartilage in joints, repairing spinal cord injuries, improving a weakened immune system, and helping patients with neurological disorders.
As of 2025, there are 32 cell or gene therapy products approved in the U.S. for cancer, eye diseases, and rare hereditary diseases. Also, many therapies are in pre-clinical stages and are expected to enter the clinical trials pipeline. Gene therapies under development are designed to address defects in the genetic code, which cause a wide range of diseases.
A shortage of manufacturing capacity poses a massive hurdle to growth in the cell and gene therapy marketplace; therefore, major firms outsource their cell or gene therapy process development, providing lucrative opportunities for the growth of the pharmaceutical contract development and manufacturing market.
Click here to: Get Free Sample Pages of this Report
The pharmaceutical industry is largely driven by scientific discovery, development, and toxicological and clinical experience. Pharmaceutical manufacturing operations may be categorized as primary production of bulk drug substances (APIs) and manufacturing of dosage form products (formulations).
In addition to the complexity of the pharmaceutical manufacturing process, many countries globally have specific legal protections for proprietary drugs and manufacturing processes, known as intellectual property rights. When legal protections are limited or nonexistent, some companies specialize in manufacturing and marketing generic drugs. The pharmaceutical industry requires large capital investments due to the high expenses associated with R&D, regulatory approvals, manufacturing, quality assurance & control, marketing, and sales.
U.S. and Canada have extensive government regulations affecting the development and approval of drugs for commercial sale. These countries have strict requirements for good manufacturing practices to ensure the integrity of drug manufacturing operations and pharmaceutical products' quality, safety, and efficacy. These challenges have led pharmaceutical companies to partner with contract manufacturing and development organizations to develop and manufacture pharmaceuticals and biologics, driving the growth of the pharmaceutical contract development and manufacturing market.
Based on type, the North America pharmaceutical contract development and manufacturing market is segmented into pharmaceutical manufacturing services, drug development services, and biologics manufacturing services. In 2025, the pharmaceutical manufacturing services segment is expected to account for the largest share of the market. The large of this segment is attributed to factors such as the growing need to reduce manufacturing costs in the pharmaceutical industry, the requirement for high-quality bulk manufacturing, and the growing demand for generic drugs. According to the U.S. FDA, as of 2022, it is estimated that 91% of all prescriptions in the United States are filled as generic drugs, with more than 32,000 generic drugs approved.
Based on end user, the North America pharmaceutical contract development and manufacturing market is segmented into large pharmaceutical companies, small & mid-size pharmaceutical companies, and generic pharmaceutical companies. In 2025, the large pharmaceutical companies segment is expected to account for the largest share of the market. The large market share of this segment is attributed to the increasing demand from businesses worldwide, the increasing need for state-of-the-art processes and production technologies, and the continued efforts of pharmaceutical companies to reduce production costs. Additionally, with the increasing burden of diseases, the demand for drugs is increasing. Thus, to tackle the high demand, pharmaceutical companies are collaborating or partnering with CDMOs. For instance: In May 2021, Siegfried Holdings AG (Switzerland) signed an agreement with Novavax, Inc. (U.S.) to manufacture and supply Novavax's protein-based COVID-19 Vaccine. Under this agreement, Siegfried served as a non-exclusive supplier for the aseptic fill and finish of Novavax's COVID-19 vaccine NVX-CoV2373.
The U.S. is expected to register the highest CAGR during the forecast period. Factors such as drug shortages due to the emergence of the COVID-19 pandemic, the presence of large pharmaceutical and biotechnology manufacturers, biopharmaceutical R&D funding, and rising demand for cell and gene therapies in the U.S. drive the pharmaceutical contract development and manufacturing market. Growing demand for biopharmaceuticals and supportive initiatives for their adoption resulted in expanding biopharmaceutical manufacturing facilities in the U.S. For instance, in April 2022, FUJIFILM Diosynth Biotechnologies (U.S.) announced to build BioProcess Innovation Center in Research Triangle Park (U.S.). The facility will have analytical instrumentation, high throughput bioprocessing equipment, and automation technologies. Also, in March 2022, Amgen, Inc. (U.S.) announced the establishment of a biomanufacturing facility in North Carolina (U.S.). The facility is expected to be operational by 2025.
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments adopted by leading market players in the industry over the years. The key players operating in the North America pharmaceutical contract development and manufacturing market are Thermo Fisher Scientific Inc. (U.S.), Catalent, Inc. (U.S.), C.H. Boehringer Sohn AG & CO. KG. (Germany), Synoes Health, Inc. (U.S.), Curia Global, Inc. (U.S.), Cambrex Corporation (U.S.), FUJIFILM Diosynth Biotechnology (U.S.), Samsung Biologics (South Korea), Siegfried Holding AG (Switzerland), Aenova Group (Germany), WuXi Biologics (China), and Vetter Pharma International GmbH (Germany).
Report Summary:
|
Particular |
Details |
|
Number of Pages |
~150 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2022 |
|
CAGR |
7.1% |
|
Estimated Market Size (Value) |
$105.42 billion by 2032 |
|
Segments Covered |
by Type
by End User
by Country
|
|
Countries Covered |
North America (U.S., Canada) |
|
Key Companies |
Thermo Fisher Scientific Inc. (U.S.), Catalent, Inc. (U.S.), C.H. Boehringer Sohn AG & CO. KG. (Germany), Synoes Health, Inc. (U.S.), Curia Global, Inc. (U.S.), Cambrex Corporation (U.S.), FUJIFILM Diosynth Biotechnology (U.S.), Samsung Biologics (South Korea), Siegfried Holding AG (Switzerland), Aenova Group (Germany), WuXi Biologics (China), and Vetter Pharma International GmbH (Germany). |
Key questions answered in the report:
What is the focus of the North America pharmaceutical contract development and manufacturing market?
The North America pharmaceutical contract development and manufacturing market studies different types of services, such as pharmaceutical manufacturing services, drug development services, and biologics manufacturing services used by end users. The North America pharmaceutical contract development and manufacturing market studied in this report involve the analysis of various segments at country levels.
The North America pharmaceutical contract development and manufacturing market is projected to reach $ 105.42 billion by 2032 at a CAGR of 7.1% from 2025 to 2032.
Among all the types studied in the report, the pharmaceutical manufacturing services segment is expected to account for the largest share of the market in 2025. The large share of the segment is attributed to the growing need to reduce manufacturing costs, bring a new drug to the market quickly at the lowest possible cost, the requirement for high-quality bulk manufacturing, and the growing demand for generic drugs.
The growth of this market is driven by the complex manufacturing requirements of the pharmaceutical industry, manufacturers' growing inclination toward the use of cutting-edge technologies, patent expiration, increasing investments in pharmaceutical R&D, and the rising demand for generic medicines & biologics. In addition, the growing demand for cell and gene therapies and personalized medicine and growth in high potency active pharmaceutical ingredients (HPAPI) and antibody-drug conjugates (ADC) markets are expected to offer significant opportunities for the growth of the pharmaceutical contract development and manufacturing market.
The key players operating in the North America pharmaceutical contract development and manufacturing market are Thermo Fisher Scientific Inc. (U.S.), Catalent, Inc. (U.S.), C.H. Boehringer Sohn AG & CO. KG. (Germany), Synoes Health, Inc. (U.S.), Curia Global, Inc. (U.S.), Cambrex Corporation (U.S.), FUJIFILM Diosynth Biotechnology (U.S.), Samsung Biologics (South Korea), Siegfried Holding AG (Switzerland), Aenova Group (Germany), WuXi Biologics (China), and Vetter Pharma International GmbH (Germany).
The U.S. is expected to offer significant growth opportunities owing to the government initiatives to create favorable pharmaceutical drug approval programs, increase investment from existing pharmaceutical manufacturers in the country for infrastructure development, and growing investments towards biosimilar manufacturing.
























Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates