Resources
About Us
North America Molecular Diagnostics Market by Product (Reagents & Kits, Systems, Software), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (Infectious Diseases, Oncology, Neurological), End User - Forecast to 2032
Report ID: MRHC - 104959 Pages: 150 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportMolecular diagnostics help detect the targeted genetic material in human, viral, and bacterial genomes. Molecular diagnostic tests are increasingly being used in many areas, including testing infectious diseases, oncology, and clinical genetics. Advancements in molecular diagnostics will continue to improve the accuracy and speed of diagnosis and will become an essential aspect of patient-tailored interventions and therapeutics. Polymerase chain reaction (PCR) and microarray technology offer high throughput and more accurate tests. Additionally, sequencing has emerged as an alternative molecular diagnostic technology with diverse applications.
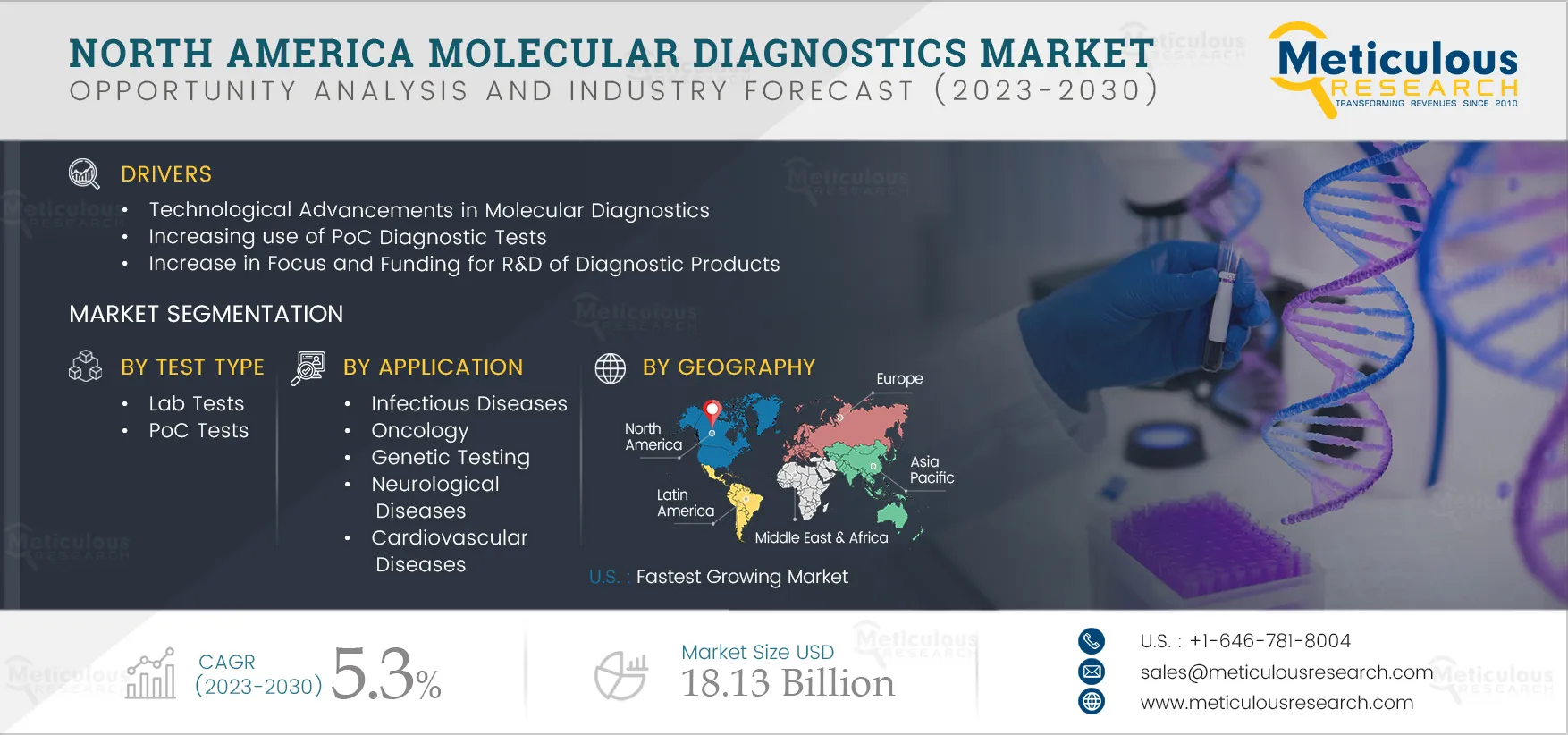
The growth of the North America molecular diagnostics market is driven by several factors, including the rising geriatric population, the increasing prevalence of communicable & non-communicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditures. However, unfavorable regulatory frameworks and the high costs of molecular diagnostic tests restrain the growth of this market.
Furthermore, increasing focus on companion diagnostics and the rising popularity of direct-to-consumer (DTC) genetic testing are expected to create growth opportunities for the players in this market. However, the shortage of skilled professionals is a major challenge for market growth. Additionally, the rising integration of artificial intelligence & machine learning is a prominent trend in the molecular diagnostics market.
Click here to: Get Free Sample Pages of this Report
The healthcare landscape is constantly changing due to innovations in medical technologies. In recent years, companies have focused on developing advanced diagnostic kits that yield faster results than traditional kits. These advanced kits allow healthcare providers and caregivers to make crucial medical decisions promptly. For instance, companies have been developing ultra-fast PCR kits to minimize the time required to generate test results.
Moreover, traditional PCR tests have transitioned to incorporate multiplex capabilities, including simultaneously detecting two or more pathogens. Multiplex diagnostic tests provide higher accuracy for critical care, which leads to pathogen-specific treatment. Also, the CLIA-waived molecular PoC platform is widely accepted, as traditional POC tests lack sensitivity.
Traditional tests can be replaced by rapid molecular diagnostic tests that are simple, easy to use, and highly accurate. Market players are launching PoC molecular PCR tests. For instance, in June 2021, Thermo Fisher Scientific (U.S.) developed the innovative and portable Accula SARS-CoV-2 Test offering gold-standard Reverse Transcription Polymerase Chain Reaction (RT-PCR) detection of SARS-CoV-2 in a point-of-care format. The test delivers results in 30 minutes, whereas the conventional RT PCR method takes around 2–3 days to produce results. The product uses a cartridge (lateral flow technology) that can be directly used to analyze samples, unlike conventional RT PCR tests, where nucleic acid extraction takes a significant amount of time.
Companion molecular diagnostics measure the levels of proteins, genes, or specific mutations to reveal specific and effective therapies for an individual’s condition. Companion diagnostics (CDx) are a form of personalized, stratified, and precision medicine that individualizes a patient’s treatment. CDx has expanded from oncology drugs to multiple therapeutic areas, and the number of combinations has grown significantly over the years.
Furthermore, personalized medicine for infectious diseases helps to orient the molecular management of infections. Molecular microbiology offers technologies that can detect and identify microorganisms rapidly. Determining the pharmacogenetic profiles of patients suffering from infectious diseases provides an additional assessment of the drug metabolizer phenotype or the risk of potential adverse drug interactions. Personalized medicine is thus expected to develop new therapies and new methods for disease detection.
Companies in this market are collaborating for the development and commercialization of CDx due to the growth potential of companion diagnostics. For instance, in September 2021, Illumina, Inc. (U.S.) and Merck KGaA (Germany) partnered to develop and commercialize companion diagnostic tests to identify genetic mutations in patients with HRD-positive tumors who are eligible for the PARP inhibitor targeted treatment.
Based on product & service, the North America molecular diagnostics market is segmented into kits & reagents, instruments, and software & services. In 2025, the kits & reagents segment is expected to account for the largest share of the market. The large market share of this segment is attributed to factors such as the commercial availability of a diverse range of diagnostic reagents & consumables, the availability of disease-specific test kits & assays, growing awareness regarding early disease diagnosis, and the outbreak of COVID-19. Moreover, the emergence of various POC tests and assays is creating significant opportunities for suppliers of molecular diagnostic kits & reagents.
Based on technology, the North America molecular diagnostics market is segmented into polymerase chain reaction (PCR), in situ hybridization (ISH), isothermal nucleic acid amplification technology (INAAT), microarrays, mass spectrometry, sequencing, and other technologies. The sequencing segment is expected to register the highest CAGR during the forecast period. The growth of this segment is attributed to the advantages of sequencing, including the detection of low-frequency variants with higher sensitivity rates, comprehensive genomic coverage, detection at a lower limit of samples, and simultaneous sequencing of multiple genes or gene regions. In January 2020, the Centers for Medicare & Medicaid Services (CMS) approved the use of Medicare coverage of next-generation sequencing tests for inherited ovarian and breast cancers, expanding the coverage of NGS as a diagnostic tool in the U.S.
Based on test type, the North America molecular diagnostics market is segmented into laboratory tests and PoC tests. In 2025, the laboratory tests segment is expected to account for the largest share of the market. The large market share of this segment is attributed to the wide availability of laboratory tests in hospitals, diagnostic laboratories, academic institutions, and research centers. Furthermore, laboratory tests are often the preferred choice among patients for diagnostic purposes. Companies’ efforts to launch new solutions for laboratory tests also contribute to the segment’s growth. For instance, in June 2022, Becton, Dickinson and Company (U.S.) launched its BD MAX Respiratory Viral Panel (RVP) assay for the BD MAX System. This RT-PCR assay is a combination test for SARS-CoV-2, Influenza A + B, and Respiratory Syncytial Virus (RSV).
Based on application, the North America molecular diagnostics market is segmented into infectious diseases, oncology, genetic testing, neurological diseases, cardiovascular diseases, and other applications. The oncology segment is expected to register the highest CAGR during the forecast period. The increasing prevalence of cancer, the rising mortality rate, and the need for early diagnosis drive the segment’s growth. In the U.S., the overall number of cancer cases is estimated to reach 2,727,182 in 2032 from 2,281,658 in 2020. (Source: GLOBOCAN). The product approval for the oncology molecular diagnostic tests also supports the market growth. For instance, in October 2020, F. Hoffmann-La Roche Ltd (Switzerland) received U.S. Food and Drug Administration (FDA) approval for the cobas EGFR Mutation Test v2. This real-time PCR test is used to qualitatively detect EGFR gene mutations in non-small cell lung cancer (NSCLC) patients.
Based on end user, the North America molecular diagnostics market is segmented into hospitals & clinics, diagnostic laboratories, academic & research institutes, and other end users. In 2025, the hospitals & clinics segment is expected to account for the largest share of the market. The large market share of this segment is attributed to the increased number of hospitalizations due to various diseases requiring molecular diagnosis and the proliferation of hospitals and clinics in emerging countries, leading to growth in the utilization of molecular diagnostic products. For instance, in 2021, hospital expenditures in the U.S. grew by 4.4% compared to 2020, reaching USD 1,323.9 billion.
The U.S. is expected to register the highest CAGR during the forecast period. The market growth in the U.S. is attributed to the large number of laboratory tests performed annually in the country, the country’s highly developed healthcare infrastructure, the rising prevalence of chronic diseases, the aging population, and the presence of large diagnostics companies. For instance, according to the American Association for Clinical Chemistry, around 13 billion laboratory tests are performed annually in the U.S. Moreover, the demand for molecular diagnostics has increased significantly since the emergence of the COVID-19 pandemic.
Key Players
The report provides a comprehensive competitive landscape analysis based on the product offerings and geographical presence of prominent market players. It also highlights key growth strategies adopted by these leading companies between 2020 and 2025. The key players operating in the North America molecular diagnostics market are Hologic, Inc. (U.S.), Danaher Corporation (U.S.), BioMérieux S.A. (France), Becton, Dickinson and Company (U.S.), Siemens Healthineers AG (Germany), DiaSorin S.p.A. (Italy), Abbott Laboratories (U.S.), Thermo Fisher Scientific Inc. (U.S.), Agilent Technologies, Inc. (U.S.), Illumina, Inc. (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), and Seegene, Inc. (South Korea).
Key questions answered in the report:
The North America Molecular Diagnostics Market study covers the market sizes & forecasts for various molecular diagnostics products used in the healthcare sector. The report involves the value analysis of various segments and subsegments of the molecular diagnostics market at the country level.
The North America Molecular Diagnostics Market is projected to reach $18.13 billion by 2032 at a CAGR of 5.3% from 2025 to 2032.
Based on product & service, in 2025, the reagents/kits segment is expected to account for the largest share of the North America Molecular Diagnostics Market. This segment is also projected to register the highest CAGR during the forecast period. The growth of this segment is driven by the increased demand for kits due to the growing prevalence of diseases and the growing awareness of early diagnosis.
The diagnostic laboratories segment is slated to register the highest CAGR during the forecast period. The growth of this segment is driven by the rising number of diagnostic laboratories and the increasing installation of advanced systems in laboratories.
The growth of the North America Molecular Diagnostics Market is driven by several factors, including the rising geriatric population, the increasing prevalence of communicable & non-communicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditures. Furthermore, increasing focus on companion diagnostics and the rising popularity of direct-to-consumer (DTC) genetic testing are expected to create growth opportunities for the players in this market.
The key players operating in the North America Molecular Diagnostics Market are Hologic, Inc. (U.S.), Danaher Corporation (U.S.), BioMérieux S.A. (France), Becton, Dickinson and Company (U.S.), Siemens Healthineers AG (Germany), DiaSorin S.p.A. (Italy), Abbott Laboratories (U.S.), Thermo Fisher Scientific Inc. (U.S.), Agilent Technologies, Inc. (U.S.), Illumina, Inc. (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), and Seegene, Inc. (South Korea).
The U.S. is expected to offer significant growth opportunities due to the high number of laboratory tests performed annually in the country, the country’s highly developed healthcare infrastructure, the rising prevalence of chronic diseases, the aging population, and the presence of large diagnostics companies.
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates