Resources
About Us
Monoclonal Antibodies Market Size, Share, Forecast, & Trends Analysis by Type (Therapeutic, Research, Diagnostic) Application (Oncology, Immunology, Cardiology, Neurology), Source (Humanized, Murine, Human), Production, End User - Global Forecast to 2032
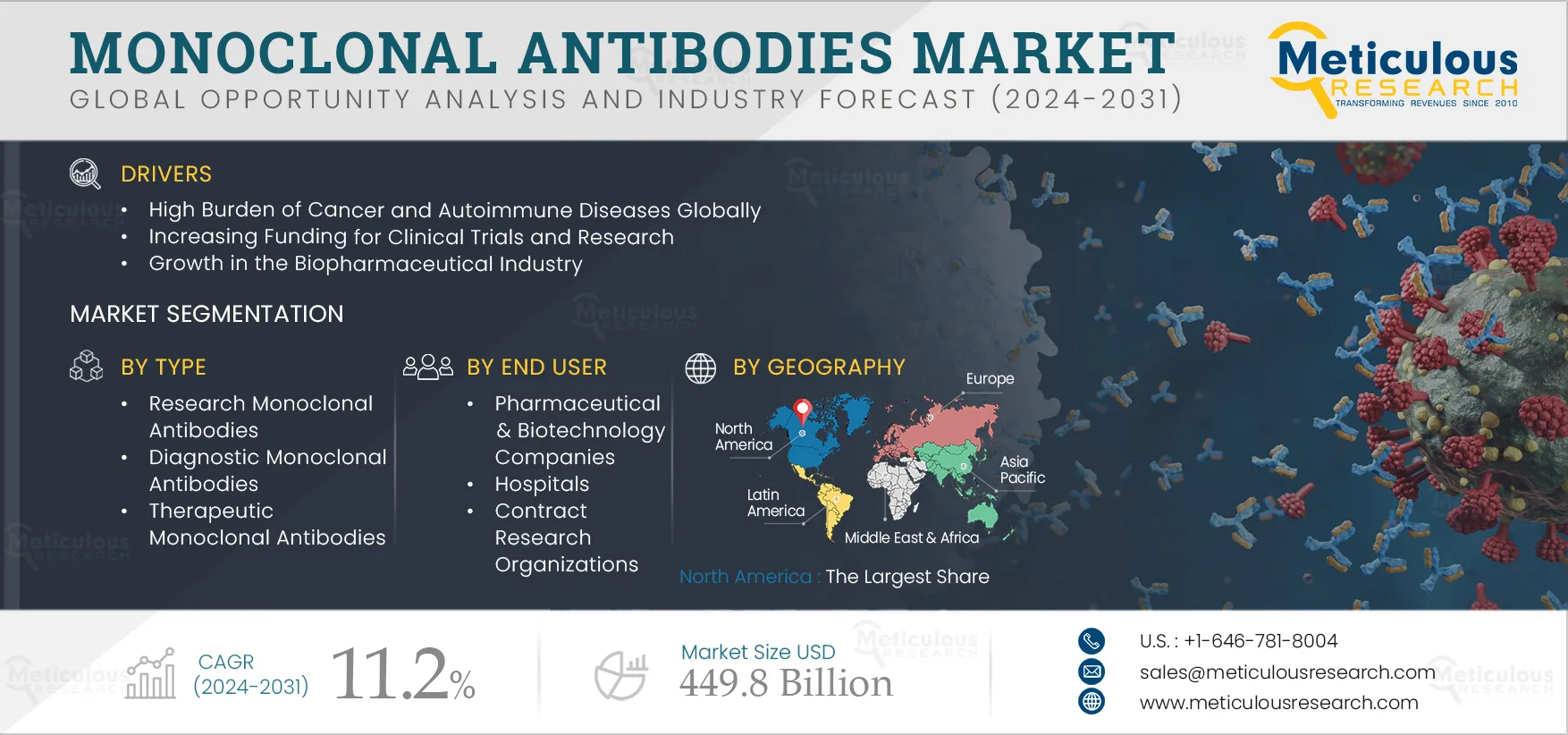
Report ID: MRHC - 1041246 Pages: 410 Jun-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Monoclonal Antibodies Market is projected to reach $449.8 billion by 2032 at a CAGR of 11.2% from 2025 to 2032. The growth of this market is driven by the high burden of cancer and autoimmune diseases globally, increasing funding for clinical trials and research, growth in the biopharmaceutical industry, rising prevalence of infectious diseases, and increasing research in proteomics and genomics.
Furthermore, increasing R&D investments by pharmaceutical and biotech companies, expanding applications of antibodies in clinical trials and medical research, increasing research activities in developing countries, the rising need for new biomarker identification, and initiatives aimed at enhancing diagnostic testing are expected to create market growth opportunities.
Cancer poses a major burden on the global population. For instance, the total number of new cancer cases is estimated to reach 24.6 million by 2032 from 19.3 million in 2020 (Source: International Agency for Research on Cancer). Monoclonal antibodies (mABs) are used for the treatment of cancer as mABs. These targeted drugs identify specific proteins and cancer cells and help in the inhibition of their growth.
Additionally, monoclonal antibodies are used to treat autoimmune diseases, a condition in which the body's immune system attacks its tissues, causing inflammation, damage to healthy tissues, and sometimes organ dysfunction. Several anti-inflammatory monoclonal antibodies are used in the treatment of autoimmune diseases. For instance, monoclonal antibodies used to treat arthritis are known as tumor necrosis factor (TNF) inhibitors. TNF is a protein involved in causing inflammation and damage of rheumatoid arthritis.
The market for biopharmaceuticals is increasing due to the increasing number of bio-approvals, companies’ rising emphasis on expanding biomanufacturing capacities, and government initiatives supporting bioproduction. In 2021, 5,500 new planned clinical trials have started, an increase of 14% from 2020 and 19% from 2019.
Government agencies worldwide are undertaking initiatives to support the growth of the biopharmaceutical market. For instance, the Chinese government has undertaken several initiatives for the development and commercialization of biosimilars. Countries like Malaysia, Indonesia, Thailand, and Taiwan have established regulatory pathways for biosimilars, increasing access to biosimilar technology. The growth in the biopharmaceutical industry is driving the development of monoclonal antibodies (mAbs). mAbs are regarded as one of the fastest-growing classes of biopharmaceuticals.
 Click here to: Get Free Sample Pages of this Report
Click here to: Get Free Sample Pages of this Report
Personalized/precision medicine uses an individual’s genomic information to offer targeted treatments. As precision medicine is based on individuals’ genetic makeup, it can overcome the limitations of traditional medicines and provide effective treatment. Monoclonal antibody therapies have improved precision medicine by providing personalized treatment options and enabling targeted therapy. These antibodies can be tailored to precisely bind and recognize disease-associated molecules, such as viral antigens or tumor markers. This targeted approach helps in the development of personalized therapies designed for individual patients, minimizing side effects and maximizing treatment efficacy.
The rising funding in R&D of personalized medicines, key players’ focus on manufacturing personalized medicines, and growing approvals for personalized medicines are driving the growth of the monoclonal antibodies market. For instance, in 2022, the U.S. FDA approved 34% of personalized medicine among total FDA approvals, marking an increase from 25% in 2019.
Antibody engineering techniques have expanded and enhanced the application of monoclonal antibodies. Researchers can optimize and modify antibodies to improve their half-life, efficacy, and stability. Some of the advancements in antibody engineering include computational antibody design, phage display technology, single B cell sequencing, CRISPR/Cas9 technology, antibody-drug conjugates (ADCS) technology, humanized mouse models, high-throughput screening (HTS), and artificial intelligence & machine learning.
AI and machine learning are now increasingly used in antibody engineering to guide the design of novel antibody formats, optimize antibody sequences for improved properties, and tools to predict antibody-antigen interactions. These approaches are becoming more useful in discovering novel therapeutic targets and optimizing the antibody discovery process. Similarly, humanized mouse models, engineered to express human antibody repertoires, are used for monoclonal antibody discovery and development. These models help for the maturation of human antibodies and in vivo generation in response to antigen challenge, thus mimicking human immune response and reducing the chances of immunogenicity observed with murine-derived antibodies.
Pharmaceutical and biotech companies are heavily investing in research and development with a focus on developing novel drugs, drug molecules, and biologics. For instance, according to the PhRMA (Pharmaceutical Research and Manufacturers of America) annual membership survey 2022, the R&D spending of PhRMA member companies in the U.S. pharmaceutical & biopharmaceutical sector reached USD 102.3 billion in 2021, a significant increase from USD 83.0 billion in 2019.
Additionally, according to PhRMA and the National Venture Capital Association, venture capital investments from established biopharmaceutical companies in emerging biotechnology firms in the U.S. to support R&D increased from USD 11 billion in 2015 to USD 15 billion in 2020.
Research & development aids in the identification and validation of new biologics, including monoclonal antibodies for therapeutic applications. Therefore, such investments in R&D are expected to lead to the development of new monoclonal antibodies, which will boost their production for therapeutic purposes.
Based on type, the monoclonal antibodies market is segmented into research monoclonal antibodies, diagnostic monoclonal antibodies, and therapeutic monoclonal antibodies. In 2025, the therapeutic monoclonal antibodies segment is expected to account for the largest share of 90.2% of the monoclonal antibodies market. This segment’s large market share can be attributed to factors such as the wide range of applications of monoclonal antibodies in therapeutics for various diseases and conditions, the increasing prevalence of chronic diseases, and the growing focus of pharmaceutical and biopharmaceutical companies on the development of monoclonal antibody-based therapeutics. Furthermore, recent approvals for therapeutic monoclonal antibodies contribute to the growth of this segment. For instance, in August 2024, Regeneron Pharmaceuticals, Inc. (U.S.) received approval for Veopoz (pozelimab-bbfg) for the treatment of adult and pediatric patients one year of age and older with CHAPLE disease. Similarly, in August 2024, Pfizer Inc. (U.S.) received approval for ELREXFIO (elranatamab-bcmm) for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM).
Based on production process, the monoclonal antibodies market is segmented into in vivo process and in vitro process. In 2025, the in vitro process segment is expected to account for the largest share of the monoclonal antibodies market. In vitro antibody production methods involve the use of recombinant technology, hybridoma cells, or mammalian cell culture to produce monoclonal antibodies. This segment's large market share can be attributed to benefits offered by in vitro production methods, including the production of specific antibody isotypes, the generation of antibodies with uniformity and high specificity, reduced reliance on mouse models through the use of humanized mouse models, and a reduced risk of immunogenicity.
Based on source, the monoclonal antibodies market is segmented into human, humanized, murine, chimeric, and other sources. In 2025, the human segment is expected to account for the largest share of 33.2% of the monoclonal antibodies market. The increased utilization of human cell lines for monoclonal antibody production, which yields antibodies most similar to those naturally produced in the human body, is the primary factor contributing to the segment’s large market share. Furthermore, the increasing number of drug launches and product approvals for human monoclonal antibodies, enhanced efficacy, improved safety profiles, and the use of humanized animal models for the production of research & diagnostic monoclonal antibodies (MABs) contribute to this segment’s large market share.
Based on end user, the monoclonal antibodies market is segmented into pharmaceutical & biotechnology companies, hospitals, contract research organizations, and other end users. In 2025, the hospitals segment is expected to account for the largest share of the monoclonal antibodies market. This segment’s large market share can be attributed to the rising number of patient visits to hospitals seeking various treatments, including monoclonal antibody therapy, the availability of dedicated facilities for the administration of monoclonal antibody drugs and access to advanced technological resources, and the proliferation of hospitals offering monoclonal antibody therapy. For instance, starting in February 2022, Northern Arizona Healthcare (U.S.) began offering monoclonal antibody treatment for COVID-19 patients at no cost at dedicated facilities in Flagstaff and Cottonwood.
In 2025, North America is expected to account for the largest share of 34.9% of the monoclonal antibodies market. North America's significant market share can be attributed to various factors, including the increasing prevalence of target diseases such as cancer and cardiovascular diseases, the increasing funding allocated for the development of monoclonal antibodies, the presence of key market players in the region, the rising number of clinical trials for monoclonal antibodies, and favorable reimbursement policies. For instance, in June 2024, the Federal Emergency Management Agency (U.S.), an agency of the U.S. Department of Homeland Security, allocated USD 50 million as reimbursement to the Commonwealth of Massachusetts for the cost of delivering monoclonal antibody treatments to the public during the COVID-19 pandemic.
However, the market in Asia-Pacific is projected to register the highest growth rate of 13.4% during the forecast period. Countries in the region, including China, India, and South Korea, are expected to offer significant opportunities for market growth. The market growth in Asia-Pacific is driven by factors such as the expanding pharmaceutical and biotech industry, supportive government initiatives, the increasing number of clinical trials conducted in the region, and the rising demand for biologics to treat chronic and infectious diseases. Additionally, key players are ramping up production and receiving approvals for monoclonal antibody production in Asia Pacific countries, driven by the growing demand and usage in the region. For instance, in January 2025, AstraZeneca plc (U.K.) and Sanofi S.A. (France) received approval for Beyfortus (nirsevimab) in China for the prevention of respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI) in neonates and infants. Furthermore, in November 2024, Aragen Life Sciences (India) invested USD 30 million to establish a biologics manufacturing facility in India, focusing on the production of monoclonal antibodies, fusion proteins, and therapeutic proteins, thereby enhancing the company’s production capacity.
The report offers a competitive landscape based on an extensive assessment of the product offerings and geographic presence of leading market players and the key growth strategies adopted by them over the past few years (2020–2025). The key players operating in the global monoclonal antibodies market are Novartis AG (Switzerland), Pfizer Inc. (U.S.), F. Hoffmann-La Roche AG (Switzerland), AbbVie Inc. (U.S.), Amgen Inc. (U.S.), Bristol-Myers Squibb (U.S.), GSK plc (U.K.), Merck KGaA (Germany), Eli Lilly and Company (U.S.), AstraZeneca plc (U.K.), Johnson & Johnson (U.S.), Takeda Pharmaceutical Company Limited (Japan), Daiichi Sankyo Company (Japan), Biogen (U.S.), and Thermo Fisher Scientific Inc. (U.S.).
In March 2025, F. Hoffmann-La Roche AG (Switzerland) launched Vabysmo (faricimab), a bispecific monoclonal antibody, in India for the treatment of diabetic macular edema (DME) and neovascular or wet age-related macular degeneration (nAMD). With this launch, the company expanded its product portfolio in monoclonal antibodies for the ophthalmology therapeutic area.
In December 2024, KBI Biopharma, a JSR Life Sciences company (U.S.), launched SUREmAb, a MAB solution based on KBI’s SUREtechnology Platform designed for cost-efficient, safe, and optimized monoclonal antibody (mAb) manufacturing and development.
|
Particulars |
Details |
|
Number of Pages |
410 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2024 |
|
CAGR |
11.2% |
|
Estimated Market Size (Value) |
$449.8 billion by 2032 |
|
Segments Covered |
By Type
By Production Process
By Source
By End User
|
|
Countries Covered |
North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, Belgium, and Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Australia, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, Rest of Latin America), and the Middle East & Africa |
|
Key Companies |
The key players operating in the global monoclonal antibodies market are Novartis AG (Switzerland), Pfizer Inc. (U.S.), F. Hoffmann-La Roche AG (Switzerland), AbbVie Inc. (U.S.), Amgen Inc. (U.S.), Bristol-Myers Squibb (U.S.), GSK plc (U.K.), Merck KGaA (Germany), Eli Lilly and Company (U.S.), AstraZeneca plc (U.K.), Johnson & Johnson (U.S.), Takeda Pharmaceutical Company Limited (Japan), Daiichi Sankyo Company (Japan), Biogen (U.S.), and Thermo Fisher Scientific Inc. (U.S.) |
The Monoclonal Antibodies Market focuses on therapeutic and diagnostic monoclonal antibodies, vital for treating diseases like cancer, autoimmune disorders, and infections, driven by advanced biopharmaceutical research.
The Monoclonal Antibodies Market is projected to reach approximately $449.8 billion by 2032, showcasing robust growth driven by increasing disease prevalence and advancements in antibody research and production.
The market is expected to grow at a CAGR of 11.2% from 2025 to 2032, driven by rising funding for clinical trials, increasing applications in medicine, and the demand for personalized therapies.
As of 2024, the Monoclonal Antibodies Market is valued significantly, with projections indicating it will expand to about $449.8 billion by 2032 due to increasing healthcare demands.
Key players include Novartis AG, Pfizer Inc., F. Hoffmann-La Roche AG, AbbVie Inc., Amgen Inc., and Bristol-Myers Squibb, among others, leading in innovation and market presence.
The market trend is shifting towards personalized medicine, emphasizing precision therapies based on individual genetics, alongside advancements in antibody engineering and production techniques.
Major drivers include the high burden of cancer and autoimmune diseases, increased R&D funding, growth in the biopharmaceutical sector, and the rising demand for effective treatment options.
Segments include therapeutic, diagnostic, and research monoclonal antibodies; production processes (in vivo and in vitro); sources (human, humanized, murine); and end-users like hospitals and biotech companies.
The global outlook is positive, with substantial growth anticipated in North America and Asia-Pacific, driven by increasing investments, technological advancements, and a rising patient population needing innovative treatments.
The Monoclonal Antibodies Market is experiencing significant growth, projected to reach $449.8 billion by 2032, fueled by escalating investments in research and a growing patient base worldwide.
The market is projected to grow at a CAGR of 11.2% from 2025 to 2032, driven by the rising burden of diseases and advancements in therapeutic antibody development.
North America is expected to hold the highest market share in 2025, accounting for 34.9% of the market, driven by a high prevalence of diseases, significant R&D funding, and a strong pharmaceutical presence.
























Published Date: Mar-2024
Published Date: Jul-2024
Published Date: Jul-2022
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates