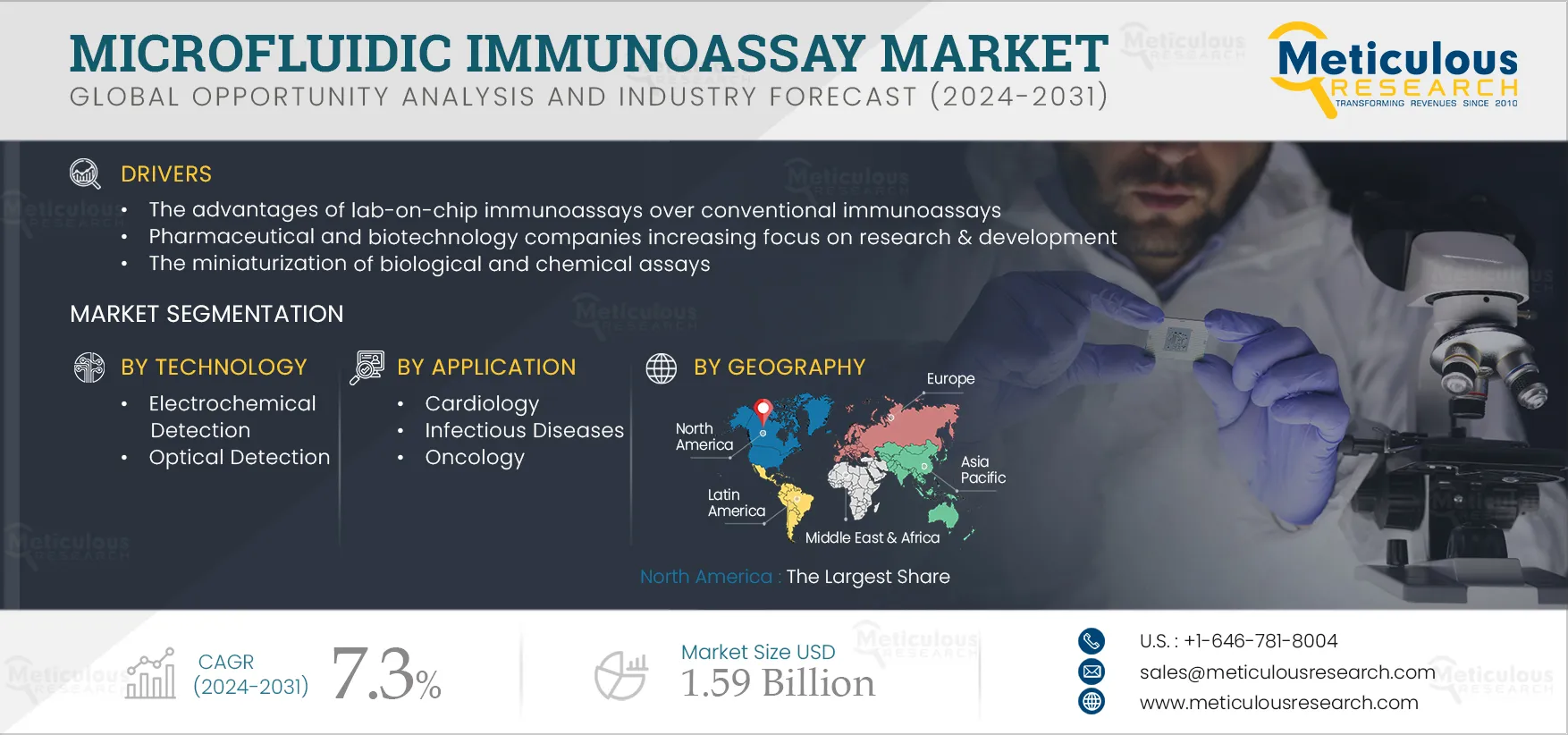
The Microfluidic Immunoassay Market is projected to reach $1.59 billion by 2031, at a CAGR of 7.3% during the forecast period. Microfluidic immunoassays use a lab-on-chip microfluidic platform to conduct the immunoassays for diagnostic and research purposes. These lab-on-chips are majorly used in point-of-care testing. Microfluidic immunoassay has major applications in the detection of biomarkers for cardiology, oncology, infectious diseases, diabetes, and other applications.
The growth of this market is driven by the increasing use of microfluidic immunoassays in drug discovery & development and biomarker detection, the advantages of lab-on-chip immunoassays over conventional immunoassays, pharmaceutical & biotechnology companies’ increasing focus on research & development, and the miniaturization of biological and chemical assays. However, bubble formation and dead volume in microfluidics devices restrain the growth of this market.
Moreover, the increasing demand for point-of-care diagnostics and recent advancements in microfluidic immunoassay technology are expected to generate market growth opportunities. However, the selection of suitable substrate materials for designing microfluidic chips and product design and cost-related limitations in developing countries are major challenges for market stakeholders.
Here are the top 10 companies operating in the Microfluidic Immunoassay Market
Incorporated in 1900 and headquartered at Chicago, Illinois, the U.S.; Abbott Laboratories is engaged in the discovery, development, manufacture, and sale of wide range of health care products. Abbott Laboratories has four reportable segments: established pharmaceutical products, diagnostic products, nutritional products, and cardiovascular and neuromodulation products.
The company has strong presence in North America, Europe, Asia-Pacific, and rest of the world.
Revvity, Inc. (U.S.) (Formerly PerkinElmer, Inc.)
Founded in 2023 and headquartered in Massachusetts, U.S., Revvity, Inc. is a multinational company that offers products, solutions, and services for the life sciences, diagnostics, and applied markets. It operates through two reportable business segments: Life Science and Diagnostics. The company offers microfluidic immunoassay products through life science business segments. The life science segment offers products for cell, gene, and protein research and drug development.
Revvity sells products and services predominantly through its distributors. Some of its subsidiaries are Caliper Life Sciences (U.K.), PerkinElmer Pty. Ltd. (Australia), and PerkinElmer do Brasil Ltda. (Brazil), PerkinElmer Instruments (Shanghai) Co. Ltd. (China), PerkinElmer SAS (France), PerkinElmer Technologies GmbH & Co. KG (Germany), PerkinElmer Srl (Italy), PerkinElmer (India) Pvt Ltd. (India), and PerkinElmer Sverige AB (Sweden).
Siemens Healthineers AG (Germany)
Founded in 1847 and headquartered in Erlangen, Germany, Siemens Healthineers AG provides healthcare solutions and services. The company operates in four segments: Imaging, Diagnostics, Varian, and Advanced Therapies.
The company operates in the microfluidic immunoassay market through the Diagnostics segment. This segment comprises in vitro diagnostic products and services for laboratory, molecular, and point-of-care diagnostics.
The company has a direct presence in over 70 countries, with main manufacturing sites in the U.S., China, and Germany. The company produces, develops, and sells various therapeutic and diagnostic services and products to healthcare providers in over 180 countries.
QuidelOrtho Corporation (U.S.)
Founded in 1979 and headquartered in California, U.S., QuidelOrtho Corporation develops and manufactures diagnostic solutions. The company operates through four business segments: Labs, Molecular Diagnostics, Point of Care, and Transfusion Medicine. It operates in the microfluidic immunoassay market through the Point of Care segment. This segment consists of instruments and tests which provide rapid results. These tests can be performed by professional healthcare providers or in a home-care setting. Further, these tests also run on portable POC analyzers.
The company has primary distribution centers in North America and Europe. Some of its subsidiaries are Ortho-Clinical Diagnostics France SAS (France), Quidel Ireland Limited (Ireland), Ortho-Clinical Diagnostics K.K. (Japan), Ortho-Clinical Diagnostics Mexico Operations S de RL de CV (Mexico), Ortho-Clinical Diagnostics (U.K.), and Quidel Corporation (U.S.).
Nanomix, Inc. (U.S.)
Founded in 2021 and headquartered in California, U.S., Nanomix, Inc. is involved in the development of analytical detection devices for point-of-care diagnostic applications. In June 2021, the company was formed with the merger of Nanomix, Inc. and Boston Therapeutics (U.S.).
The company provides a Nanomix System (eLab) with a handheld reader and a microfluidic cartridge with a carbon nanostructure biosensor. It is used in emergency departments and by pre-hospital first responders. The system is used to diagnose myocardial infarction and manage infectious diseases.
The company has a distribution network spread across Europe, Southeast Asia, and the Middle East, with a manufacturing facility in the U.S. The company has partnered with RedPharm (Beijing) Biotechnology Co., Ltd. (China) to produce and distribute microfluidic cartridges for the S1 panel assay.
Becton, Dickinson and Company (U.S.)
Founded in 1897 and headquartered in New Jersey, U.S., Becton, Dickinson and Company is a medical technology firm engaged in developing, manufacturing, and selling various medical devices, supplies, laboratory equipment, and diagnostic products. The company offers its products to healthcare institutions, life science researchers, physicians, clinics, diagnostic laboratories, and the pharmaceutical industry.
BD operates through three reportable business segments: BD Medical, BD Life Sciences, and BD Interventional. The BD Life Sciences segment comprises two organizational units: Integrated Diagnostic Solutions and Biosciences. The company operates in the microfluidic immunoassay market through bioscience segments.
The company has manufacturing facilities outside the U.S. in Bosnia and Herzegovina, Brazil, Canada, China, Dominican Republic, France, Germany, Hungary, India, Ireland, Italy, Japan, Mexico, the Netherlands, Singapore, Spain, and the U.K. Its subsidiaries include Becton Dickinson Canada Inc., Becton Dickinson GmbH (Germany), BD Rapid Diagnostic (Suzhou) Co., Ltd. (China), Becton Dickinson Asia Limited (Hong Kong), Becton, Dickinson B.V. (Netherlands), and Becton Dickinson India Private Limited (India).
Micropoint Biotechnologies Co., Ltd. (China)
Founded in 2007 and headquartered in Shenzhen, China.; Micropoint Biotechnologies Co., Ltd. provides rapid point-of-care diagnostic products and self-care products. The company’s COVID-19 testing, electrochemistry, and immunofluorescence products are based on microfluidic technology.
The company has patented microfluidic immunofluorescence technology. The company offers microfluidic products for the thyroid, cardiovascular, infectious diseases, diabetes, fertility, coagulation, and veterinary.
The company offers products to the end users, including airports, hospitals, schools, offices, health centers, stations, and others. The company also provides technical training and consultation services for the products. The company has more than 100 distribution partners across the globe. The company has ISO13485, CE, and NMPA certification.
Biosurfit SA (Portugal)
Founded in 2006 and headquartered in Azambuja, Portugal, Biosurfit SA is a diagnostics company focused on the development & manufacturing of in-vitro diagnostic (IVD) tests for the point-of-care (POC) applications. The company has developed spinit technology, which is the diagnostic system capable of performing POC blood tests (such as immunoassays, hematology, and clinical chemistry) on the same instrument.
The company uses patented advanced proprietary microfluidics technology to conduct all the steps required in diagnostic testing. The company has 31 families of patents. The company also provides contract manufacturing services for the development of IVD tests and other medical diagnostic services for the point-of-care market.
The company’s brands include at-home lab testing, mylab.care, pharmacare, and spinit. The company has distributors in the Netherlands, Czech Republic, Portugal, Switzerland, Germany, Romania, Spain, and the rest of the world.
Koninklijke Philips N.V. (Netherlands)
Founded in 1891 and headquartered in Eindhoven, Netherlands, Koninklijke Philips N.V. is involved in developing, manufacturing, and distributing diagnosis and treatment solutions that support precision diagnosis and effective, minimally invasive interventions and therapy, health IT solutions, products for oral healthcare solutions, mother & childcare solutions, and personal care.
Philips operates through four reportable business segments: Personal Health, Diagnosis & Treatment, Connected Care, and Other. The Diagnosis & Treatment segment comprises Ultrasound, Diagnostic Imaging, Image Guided Therapy, and Enterprise Diagnostic Informatics. The company operates in the microfluidic immunoassay market through its Enterprise Diagnostic Informatics segment.
Koninklijke Philips N.V. has a geographical presence in more than 100 countries globally, including the U.S., Netherlands, China, Japan, the U.K., France, Germany, and other countries. The company has several manufacturing sites in North America, including New York, California, and Pennsylvania. The company has four regional innovation hubs in the U.S., India, Europe, and China.
NanoEnTek Inc. (South Korea)
Founded in 2000 and headquartered in Seoul, South Korea, NanoEnTek Inc. offers life science experiment equipment and medical in-vitro diagnostic devices, including point-of-care devices. The company is focusing on the development of platforms based on lab-on-a-chip technology.
The company has commercialized the FREND microfluidic system with a wide range of cartridges for cardiovascular, hormonal, tumor, vitamin, and inflammatory marker detection. The underlying technology of the company’s products is based on the convergence of microelectromechanical systems (MEMS) with microfluidics. The company owns about 100 patents on nano-convergence technology. The company provided microfluidic immunoassay products under the diagnostics business segment.
The company has a presence in 150 countries, along with a major presence in the U.S., China, and Korea. The company has 124 patients with 10 FDA 510 (K) approval and 44 products. The company has a network of partners, some of which are Thermo Fisher Scientific, Inc. (U.S.), PerkinElmer, Inc. (U.S.), Medline Industries (U.S.), Henry Schein, Inc. (U.S.), and others. Some of the company’s subsidiaries include NanoEnTek America, Inc. and NanoEnTek Bio-Technology (Beijing) Ltd.