Resources
About Us
Latin America Molecular Diagnostics Market by Offering (Reagents, Kits, Systems, Software, Services), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (HIV, Influenza, HPV, Oncology), End User - Forecast to 2032
Report ID: MRHC - 1041076 Pages: 220 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportMolecular diagnostics help detect the targeted genetic material in human, viral, and bacterial genomes. Molecular diagnostic tests are increasingly being used in many areas, including infectious diseases, oncology, and clinical genetics. Advancements in molecular diagnostics will continue to improve the accuracy and speed of diagnosis and will become an essential aspect of patient-tailored interventions and therapeutics.
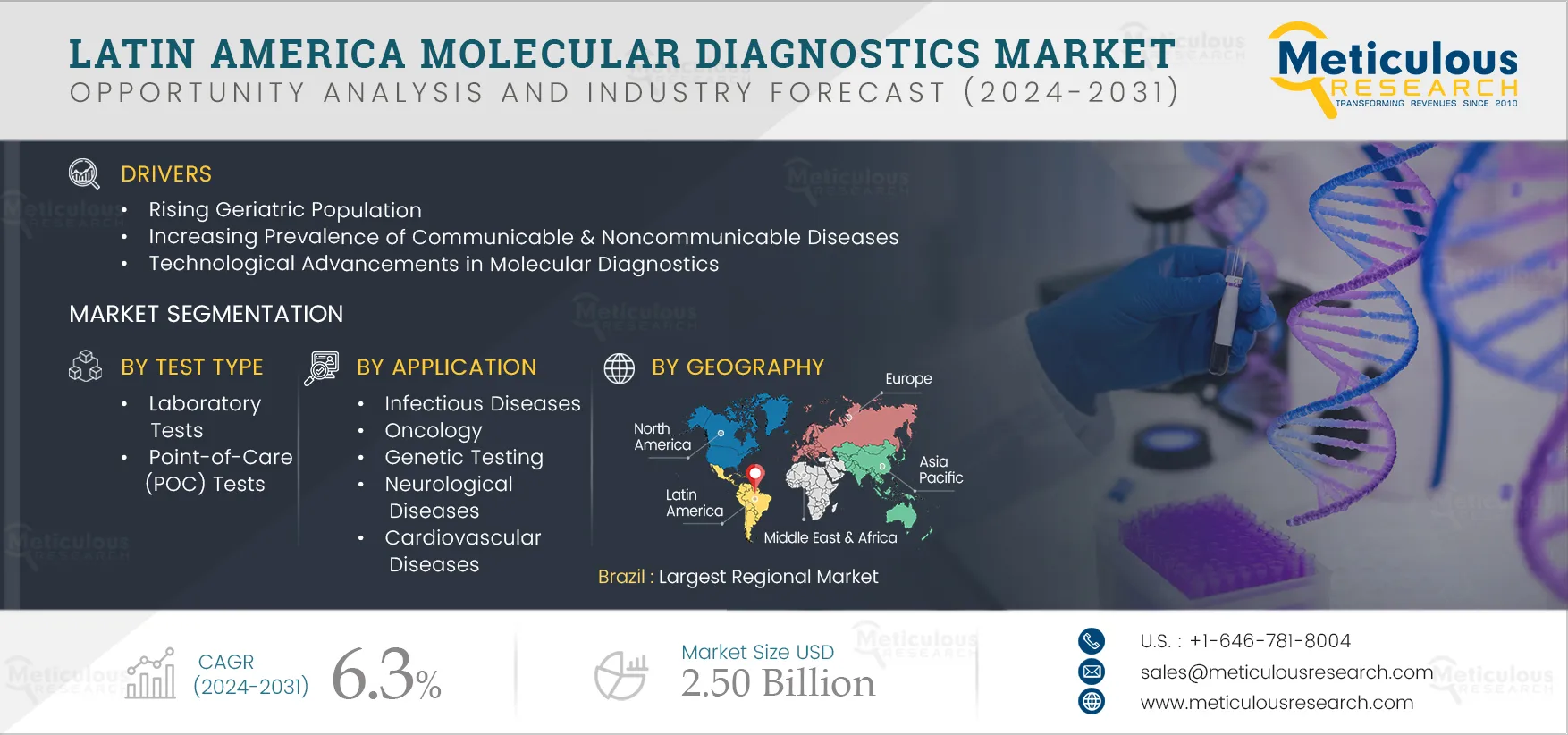
The growth of the Latin America molecular diagnostics market is driven by several factors, including the rising global geriatric population, increasing prevalence of communicable & noncommunicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditures. Factors such as growing scope in emerging economies, increasing focus on companion diagnostics, and rising popularity of direct-to-consumer (DTC) genetic testing are a few of the opportunities that would help grow the market in the future. A shortage of skilled professionals could be considered a challenge for the Latin America molecular diagnostics market. However, unfavorable regulatory frameworks and high costs of molecular diagnostic tests restrain the growth of the market.
Click here to: Get Free Sample Pages of this Report
The healthcare landscape is constantly changing due to innovations in medical technologies. In recent years, companies have been focused on developing advanced diagnostic kits that can yield faster results than traditional kits. These advanced kits allow healthcare providers and caregivers to make crucial medical decisions promptly. Several companies have been developing ultra-fast PCR kits to minimize the time required to generate test results.
For instance, in June 2021, Thermo Fisher Scientific, Inc. (U.S.) developed the innovative and portable Accula SARS-CoV-2 Test offering gold-standard Reverse Transcription Polymerase Chain Reaction (RT-PCR) detection of SARS-CoV-2 in a point-of-care format. The test delivers results in 30 minutes, whereas the conventional RT-PCR method takes around 2-3 days to produce results. The product uses a cartridge (lateral flow technology) that can be directly used to analyze samples, unlike conventional RT PCR tests, where nucleic acid extraction takes a significant amount of time.
Integrating digital technologies in clinical and ambulatory settings helps improve doctors’ and physicians’ ability to reach an initial diagnosis. Also, the digitalization of diagnostic services helps document and analyze patient data and test results, which minimizes human error and makes treatment and care pathways more consistent. Hence, an increasing number of patients’ medical histories instantly.
Companion molecular diagnostics measures the levels of proteins, genes, or specific mutations to reveal specific and effective therapies for an individual’s condition. Companion diagnostics (CDx) are a form of personalized, stratified, and precision medicine that individualizes a patient’s treatment. CDx has expanded from oncology drugs to multiple therapeutic areas, and the number of combinations has grown significantly over the years. Also, personalized medicine for infectious diseases helps to orient the molecular management of infections. Molecular microbiology offers technologies that can detect and identify microorganisms rapidly.
Determining the pharmacogenomic profiles of patients suffering from infectious diseases provides an additional assessment of the drug metabolizer phenotype and/or of the risk of potential adverse drug interactions. Personalized medicine is thus expected to help develop new therapies and new methods for disease detection.
Among offerings, in 2025, the reagents & kits segment is expected to account for the largest share of the market. Molecular diagnostic kits are widely used by healthcare professionals in hospitals, clinics, and diagnostic laboratories to diagnose and manage various communicable & non-communicable diseases. Recurrent use of assays & kits in the detection of various diseases through molecular diagnostics, rise in product approvals, and technological advancements in molecular techniques contribute to the large market share of this segment.
Among test types, in 2025, the laboratory tests segment is expected to account for the largest share of the market. Lab tests are traditionally used in hospitals and diagnostic centers and are widely accepted. The sample is taken from the patient and sent to the laboratory for processing and testing, where the technicians conduct the processing and testing and create a report for the clinician. The laboratory testing approach for molecular diagnostics has many advantages over other approaches. It has higher accuracy and reliability when compared to point-of-care testing, owing to which the adoption of molecular diagnostics in the lab is more compared to PoC tests.
Among technologies, in 2025, the polymerase chain reaction (PCR) segment is expected to account for the largest share of the molecular diagnostics market in Latin America. PCR allows rapid amplification of a specific segment of DNA. This technology is sensitive and thus helps in the diagnosis of diseases. It is effective for low-number targets and less expensive than other technologies, such as sequencing. Hence, the huge demand for PCR devices further contributes to the segment’s largest share.
Based on application, in 2025, the infectious disease segment is expected to account for the largest share of the molecular diagnostics market in Latin America. Infectious diseases are transmissible or communicable illnesses that spread quickly in some areas and become epidemics. It is crucial to quickly diagnose initial infections and prevent further spread through in vitro diagnosis. The growing prevalence of infectious diseases, the rising funds for infectious disease diagnostic research activities, and the sudden emergence of a pandemic are the factors attributed to the large market share of this segment.
Based on end users, in 2025, the hospitals & clinics segment is expected to account for the largest share of the molecular diagnostics market in Latin America. Factors contributing to the segment’s share include increased hospitalizations due to various diseases, the rise in the geriatric population, and the high prevalence of healthcare-associated infections (HAIs).
In 2025, Brazil is expected to dominate the Latin America molecular diagnostics market. Brazil has a high prevalence of infectious diseases such as hepatitis, dengue, malaria, chikungunya, and zika, which pose significant challenges to the public healthcare system. For this, the Brazilian Ministry of Health, in accordance with the WHO, has outlined a national strategy that aims to expand access to prevention, diagnosis, and treatment of hepatitis C to reduce new infections and mortality. This is expected to offer significant opportunities for the growth of molecular diagnostics in Brazil.
The key players profiled in the Latin America molecular diagnostics market report are Bio-Manguinhos (Brazil), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), OmicronLab (Mexico), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.).
The report includes a competitive landscape based on an extensive assessment of the market based on offering, test type, application, technology, and end user. The report also provides insights into the presence of major market players and their key growth strategies in the last three to four years.
Report Summary:
|
Particular |
Details |
|
Page No |
~220 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2025 |
|
CAGR |
6.3% |
|
Market Size (Value) |
$2.50 billion |
|
Market Size (Volume) |
NA |
|
Segments Covered |
By Offering
By Test Type
By Technology
By Application
By End User
|
|
Countries Covered |
Latin America (Brazil, Mexico, Colombia, Argentina, Chile, Peru, Uruguay, and Rest of Latin America) |
|
Key Companies |
Bio-Manguinhos (Brazil), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), OmicronLab (Mexico), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), Agilent Technologies, Inc. (U.S.). |
This market study covers the market sizes & forecasts of the molecular diagnostics market in Latin America based on offering, test type, technology, application, end user, and geography. This market study also provides the value analysis of various segments and subsegments of the Latin America molecular diagnostics market at country levels.
The Latin America molecular diagnostics market is projected to reach $2.50 billion by 2032, at a CAGR of 6.3% during the forecast period.
The kits & reagents segment is expected to account for the largest share of the market in 2025. Factors such as the need for kits for the growing prevalence of diseases and the growing awareness of early diagnosis among people lead to the largest share of the segment.
The infectious diseases segment is expected to account for the largest share of the market in 2025. Infectious diseases are transmissible or communicable illnesses that spread quickly in some areas and become epidemics. It is crucial to quickly diagnose initial infections and prevent further spread through in vitro diagnosis. The growing prevalence of infectious diseases, the rising funds for infectious disease diagnostic research activities, and the sudden emergence of a pandemic are the factors attributed to the large market share of the segment.
The growth of the Latin America molecular diagnostics market is driven by several factors, including the rising geriatric population, increasing prevalence of communicable & noncommunicable diseases, technological advancements in molecular diagnostics, and rising healthcare expenditures.
Furthermore, the growing scope in emerging economies, increasing focus on companion diagnostics, and the rising popularity of direct-to-consumer (DTC) genetic testing serve as major opportunities for the existing market players and new entrants in the Latin America molecular diagnostics market.
The key players profiled in the Latin America molecular diagnostics market report are Bio-Manguinhos (Brazil), F. Hoffmann-La Roche Ltd. (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Hologic, Inc. (U.S.), Illumina, Inc. (U.S.), OmicronLab (Mexico), QIAGEN N.V. (Netherlands), Danaher Corporation (U.S.), Abbott Laboratories (U.S), and Agilent Technologies, Inc. (U.S.).
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates