Resources
About Us
IVD Quality Control Market Size, Share, Forecast & Trends Analysis by Offering (Product [Serum, Blood], Data Management, Service) Technology (Biochemistry, Molecular, Immunoassay) Application (cardiology, Oncology) End User – Global Forecast to 2031
Report ID: MRHC - 104119 Pages: 267 Jan-2024 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportThe IVD quality control market covers quality control products, external quality assessment services, and data management solutions offered by companies for infectious diseases, cardiology, hematology, oncology, and other applications for use in hospitals & clinics, diagnostic laboratories, academic & research institutes, and blood banks.
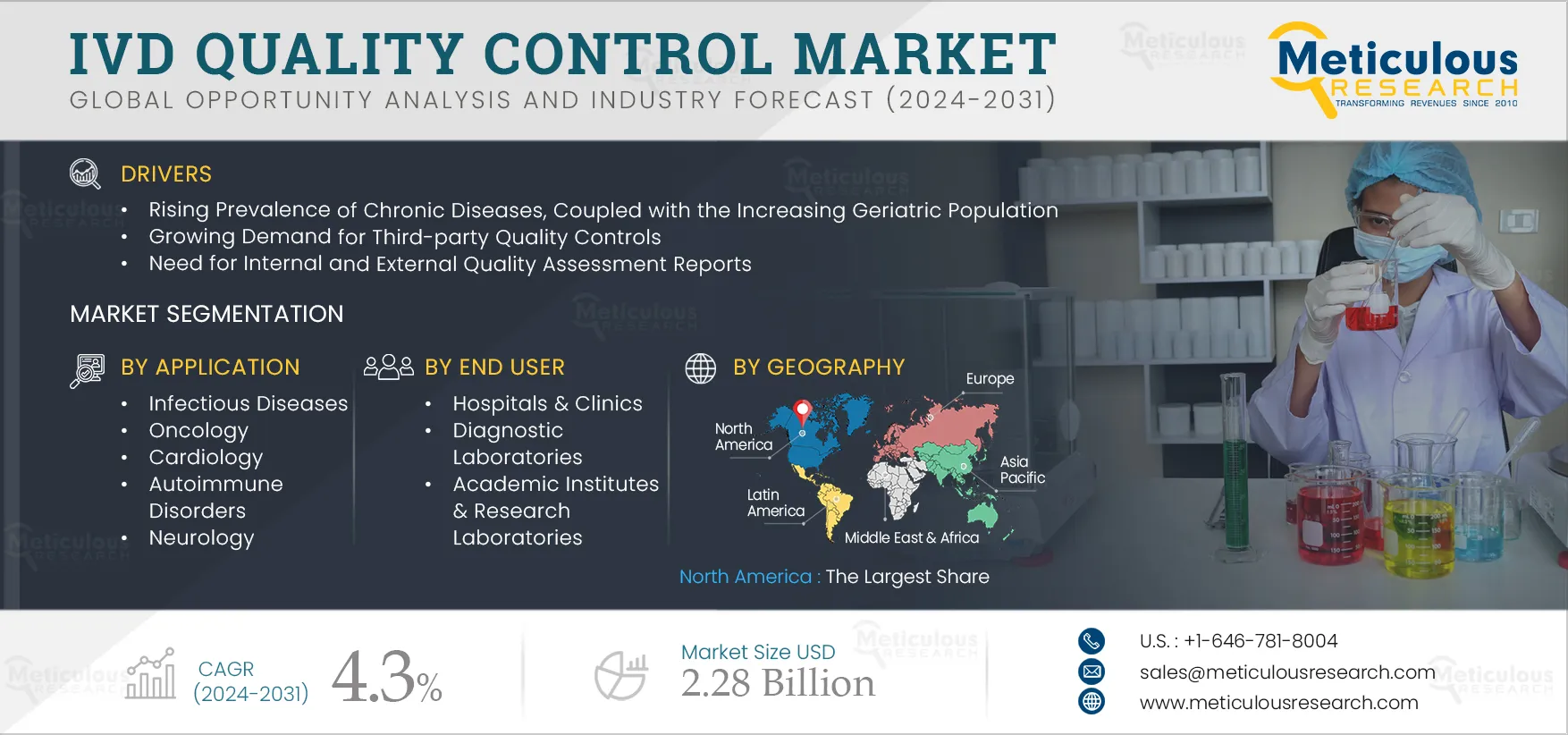
The rising prevalence of chronic diseases, coupled with the increasing geriatric population, growing demand for third-party quality controls, need for internal and external quality assessment reports, increasing number of clinical laboratories, and growing demand for Point-of-Care (POC) and rapid diagnostics drive the market growth. However, stringent technical requirements and regulatory processes for quality controls restrain this market's growth.
Among the IVD quality controls commercially available, there is an increasing demand for multi-analyte and multi-instrument controls. Multi-analyte controls are designed to contain multiple analytes of interest, representing a range of concentrations typically found in patient samples. On the other hand, multi-instrument controls are designed to be compatible with different types of laboratory instruments and testing platforms.
There is an increasing demand for multi-analyte controls as they eliminate the need to use separate controls for each analyte, streamlining the quality control process and saving time and resources. They are also cost-effective, as consolidating multiple analytes into a single control can be more cost-effective than purchasing separate controls for each analyte. Overall, the demand for multi-analyte and multi-instrument controls is driven by the need for efficient, cost-effective, and standardized quality control processes in diagnostic testing.
Click here to: Get Free Sample Pages of this Report
Both the internal and external quality assessment reports are required due to several reasons. Quality Assessment (QA) reports provide a systematic evaluation of the laboratory's performance by monitoring the accuracy and precision of test results. These reports ensure that the laboratory consistently produces reliable results. Internal QA reports contribute to meeting regulatory standards and accreditation requirements. These reports demonstrate the laboratory's commitment to quality and compliance with established guidelines. By analyzing internal QA data, laboratories can identify trends, patterns, and potential sources of error. This information facilitates targeted process improvements, leading to enhanced testing procedures and reduced chances of errors. Whereas external QA reports enable laboratories to compare their results with those of other facilities. Discrepancies can identify areas for improvement and prompt corrective actions. Moreover, participation in external QA programs is often a requirement for laboratory accreditation. Proficiency testing through external QA helps laboratories maintain their accreditation status. External QA reports validate the accuracy of a laboratory's testing methods and verify its ability to generate reliable results. These assessment data are analyzed using quality controls, hence driving the market growth.
Among the IVD quality controls commercially available, there is an increasing demand for multi-analyte and multi-instrument controls. Multi-analyte controls are designed to contain multiple analytes of interest, representing a range of concentrations typically found in patient samples. On the other hand, multi-instrument controls are designed to be compatible with different types of laboratory instruments and testing platforms. There is an increasing demand for multi-analyte controls as they eliminate the need to use separate controls for each analyte, streamlining the quality control process and saving time and resources.
Furthermore, they are also cost–effective, as consolidating multiple analytes into a single control can be more cost-effective than purchasing separate controls for each analyte. Overall, the demand for multi-analyte and multi-instrument controls is driven by the need for efficient, cost-effective, and standardized quality control processes in diagnostic testing.
Based on offering, the quality control products segment is segmented into quality control products, quality assessment services, and data management solutions. In 2024, the quality control products segment is expected to account for the largest share of 77.4% of the IVD quality control market. The large market share of this segment is attributed to the wide availability of controls for various applications. Also, advancements in these controls have expanded their utilization. For instance, in February 2020, Bio-Rad Laboratories, Inc. (U.S.) launched EDX RP Positive Run Control for clinical respiratory assays. It contains 22 respiratory analytes.
Furthermore, the quality control products segment is projected to witness the highest growth rate of 4.4% during the forecast period of 2024–2031.
Based on technology, the quality control products segment is segmented into immunoassay/immunochemistry, biochemistry/clinical chemistry, molecular diagnostics, hematology, coagulation/hemostasis, microbiology, and other technologies. In 2024, immunoassay/immunochemistry segment is expected to account for the largest share of 32.8% the IVD quality control market. The increasing use of immunoassays in POC & infectious disease testing, development of novel tests, increasing usage of miniaturized devices, and rising demand for immunoassay-based tests are expected to support the segment’s largest share. For instance, in February 2021, Agilent Technologies Inc. (U.S.) launched Agilent Dako SARS-CoV-2 IgG, an immunoassay kit to detect SARS-CoV-2 IgG antibodies.
Based on applications, the quality control products segment is segmented into infectious diseases, oncology, cardiology, autoimmune disorders, neurology, and other applications. In 2024, the infectious diseases segment is expected to account for the largest share of the IVD quality control market. There has been an increasing prevalence of infectious diseases globally. According to a 2022 article published by Families Fighting Flu, Inc. (U.S.), an estimated 1 billion people worldwide are infected by seasonal influenza every year, of which 3-5 million people have a severe case of flu. Moreover, according to the WHO, a total of 1.6 million people died from TB in 2021 globally (out of which 187,000 were HIV patients). Hence, the higher adoption of sensitive and cost-effective tests to diagnose increasing infectious diseases resulted in an increased demand for IVD quality controls for infectious diseases.
Based on end users, the quality control products segment is segmented into hospitals & clinics, diagnostics laboratories, academic & research institutes, and blood banks. In 2024, the hospitals & clinics segment is expected to account for the largest share of the IVD quality control market. The largest share of this segment is mainly attributed to the large share of diagnostics tests performed in the hospital-attached laboratories and the relatively higher demand for quality controls in these healthcare settings to ensure the accuracy and reliability of IVD test results of the patients. Additionally, during COVID-19, hospital admissions increased, which further led to the adoption of COVID-19 test quality controls in hospitals & clinics.
In 2024, North America is expected to account for the largest share of the 38.2% global IVD quality control market. The large market share of this region is attributed to the rising prevalence of chronic and infectious diseases, increasing awareness regarding early disease diagnosis, advanced diagnostics infrastructure, a large number of laboratory tests performed annually, the mandatory quality assessments of laboratories, and the participation of laboratories in external quality assessment programs.
Moreover, the market in Asia-Pacific is slated to register the highest growth rate of 5.4% during the forecast period. The rising population of the country, the increasing prevalence of disorders such as diabetes and HIV, the growth in the number of medical laboratories to increase the testing capacity of the patients and the focus of the country toward the quality assurance standards of these medical laboratories are some of the factors diving the demand for IVD quality control in China.
This report includes a competitive landscape based on an extensive assessment of the key growth strategies adopted by leading market participants in the last few years. The key players profiled in the IVD quality control market report are Seimens Healthineers AG (Germany), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), LGC Group (U.K.), Thermo Fisher Scientific Inc. (U.S.), SERO AS (Norway), Randox Laboratories Ltd. (U.K.), QuidelOrtho Corporation (U.S.), Streck LLC (U.S.), and Microbiologics, Inc. (U.S.).
|
Particular |
Details |
|
Page No |
267 |
|
Format |
|
|
Forecast Period |
2024-2031 |
|
Base Year |
2023 |
|
CAGR |
4.3% |
|
Market Size (Value) |
USD 2.28 billion by 2031 |
|
Segments Covered |
By Offering
By Technology
By Application
By End User
|
|
Countries Covered |
North America (U.S., Canada), Europe (Germany, France, Italy, U.K., Spain, and Rest of Europe), Asia-Pacific (China, Japan, India, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), Middle East & Africa |
|
Key Companies |
Seimens Healthineers AG (Germany), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), LGC Group (U.K.), Thermo Fisher Scientific Inc. (U.S.), SERO AS (Norway), Randox Laboratories Ltd. (U.K.), QuidelOrtho Corporation (U.S.), Streck LLC (U.S.), and Microbiologics, Inc. (U.S.). |
This study covers quality control products, quality assessment services, and data management solutions applied in hospitals & clinics, diagnostic laboratories, academic institutes & research laboratories, and other end users.
The IVD quality control market is projected to reach $2.28 billion by 2031, at a CAGR of 4.3% during the forecast period.
Based on offering, in 2024, the quality control products segment is expected to account for the largest share of the IVD quality control market. Developments of multi-analyte quality controls and new product launches contribute to the segment’s largest share.
Based on technology, in 2024, the immunoassay/immunochemistry segment is expected to account for the largest share of the IVD quality control market. The growing number of immunoassay/immunochemistry tests, as they provide rapid, convenient, and accurate results for the detection and quantitation of targets and the continuous development of new biomarkers, contribute to the segment’s largest share.
Based on application, in 2024, the infectious diseases segment is expected to account for the largest share of the IVD quality control market. The rising prevalence of infectious diseases and government initiatives to promote awareness and testing of these infectious diseases contribute to the segment’s largest share.
Based on end user, in 2024, the hospitals & clinics segment is expected to account for the largest share of the IVD quality control market. The expansion of hospitals, the increasing number of patients visiting the hospitals & clinics, and the collaborations between hospitals and government organizations for screening programs support the segment’s largest share.
The rising prevalence of chronic diseases, coupled with the increasing geriatric population, growing demand for third-party quality controls, need for internal and external quality assessment reports, increasing number of clinical laboratories, growing demand for Point-of-Care (POC) and rapid diagnostics are some of the factors driving the growth of this market. Additionally, the growing demand for multi-analyte and multi-instrument controls offers opportunities for market growth.
Key companies operating in the IVD quality control market are Seimens Healthineers AG (Germany), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), LGC Group (U.K.), Thermo Fisher Scientific Inc. (U.S.), SERO AS (Norway), Randox Laboratories Ltd. (U.K.), QuidelOrtho Corporation (U.S.), Streck LLC (U.S.), Microbiologics, Inc. (U.S.), and Bio-Techne Corporation (U.S.)
1. Introduction
1.1. Market Definition & Scope
1.2. Market Ecosystem
1.3. Currency & Limitations
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Approach
2.2. Process of Data Collection and Validation
2.2.1. Secondary Research
2.2.2. Primary Research/Interviews with Key Opinion Leaders of the Industry
2.3. Market Sizing and Forecast
2.3.1. Market Size Estimation Approach
2.3.2. Growth Forecast Approach
2.4. Assumptions for the Study
3. Executive Summary
4. Market Insights
4.1. Overview
4.2. Factors Affecting Market Growth
4.2.1. Impact Analysis of Market Dynamics
4.2.2. Factor Analysis
4.3. Technology Trends
4.4. Regulatory Analysis
4.4.1. Regulations for IVD Products
4.4.1.1. North America
4.4.1.1.1. U.S.
4.4.1.1.2. Canada
4.4.1.2. Europe
4.4.1.3. Asia-Pacific
4.4.1.3.1. China
4.4.1.3.2. Japan
4.4.1.3.3. India
4.4.1.4. Latin America
4.4.1.5. Middle East & Africa
4.4.2. Regulations for Quality Management
4.4.2.1. North America
4.4.2.1.1. U.S.
4.4.2.1.2. Canada
4.4.2.2. Europe
4.4.2.2.1. Germany
4.4.2.2.2. France
4.4.2.2.3. U.K.
4.4.2.2.4. Italy
4.4.2.2.5. Spain
4.4.2.2.6. Rest of Europe (RoE)
4.4.2.3. Asia-Pacific
4.4.2.3.1. China
4.4.2.3.2. Japan
4.4.2.3.3. India
4.4.2.3.4. Rest of Asia-Pacific (RoAPAC)
4.4.2.4. Latin America
4.4.2.5. Middle East & Africa
4.5. Pricing Analysis
4.6. Porter’s Five Forces Analysis
4.6.1. Bargaining Power of Buyers
4.6.2. Bargaining Power of Suppliers
4.6.3. Threat of Substitutes
4.6.4. Threat of New Entrants
4.6.5. Degree of Competition
4.7. Associated Market Analysis: In Vitro Diagnostics (IVD) Market
5. IVD Quality Control Market Assessment—by Offering
5.1. Overview
5.2. Quality Control Products
5.2.1. Quality Control Products, by Type
5.2.1.1. Serum/Plasma-based Controls
5.2.1.2. Whole Blood-based Controls
5.2.1.3. Urine-based Controls
5.2.1.4. Other Controls
5.2.2. Quality Control Products, by Function
5.2.2.1. Independent Controls
5.2.2.2. Instrument-specific Controls
5.3. Quality Assessment Services
5.4. Data Management Solutions
6. IVD Quality Control Market Assessment—by Technology
6.1. Overview
6.2. Immunoassay/Immunochemistry
6.3. Biochemistry/Clinical Chemistry
6.4. Molecular Diagnostics
6.5. Hematology
6.6. Coagulation/Hemostasis
6.7. Microbiology
6.8. Other Technologies
7. Global IVD Quality Control Market Assessment—by Application
7.1. Overview
7.2. Infectious Diseases
7.3. Oncology
7.4. Cardiology
7.5. Autoimmune Disorders
7.6. Neurology
7.7. Other Applications
8. IVD Quality Control Market Assessment—by End User
8.1. Overview
8.2. Hospitals & Clinics
8.3. Diagnostic Laboratories
8.4. Academic Institutes & Research Laboratories
8.5. Other End Users
9. IVD Quality Control Market Assessment—by Geography
9.1. Overview
9.2. North America
9.2.1. U.S.
9.2.2. Canada
9.3. Europe
9.3.1. Germany
9.3.2. France
9.3.3. U.K.
9.3.4. Italy
9.3.5. Spain
9.3.6. Rest of Europe
9.4. Asia-Pacific
9.4.1. China
9.4.2. Japan
9.4.3. India
9.4.4. Rest of Asia-Pacific
9.5. Latin America
9.5.1. Brazil
9.5.2. Mexico
9.5.3. Rest of Latin America
9.6. Middle East & Africa
10. Competition Analysis
10.1. Introduction
10.2. Key Growth Strategies
10.3. Competitive Benchmarking
10.4. Competitive Dashboard
10.4.1. Industry Leaders
10.4.2. Market Differentiators
10.4.3. Vanguards
10.4.4. Emerging Companies
11. Company Profiles (Business Overview, Financial Overview, Product Portfolio, Strategic Developments, and SWOT Analysis*)
11.1. Siemens Healthineers AG
11.2. Bio-Rad Laboratories, Inc.
11.3. Danaher Corporation
11.4. LGC Group
11.5. Thermo Fisher Scientific Inc.
11.6. Randox Laboratories Ltd.
11.7. SERO AS
11.8. QuidelOrtho Corporation
11.9. Streck LLC
11.10. Microbiologics, Inc.
11.11. Bio-Techne Corporation
12. Appendix
12.1. Available Customization
12.2. Related Reports
(Note: SWOT analyses of the top 5 companies is provided.)
List of Tables
Table 1 A Comparison Between the Characteristics of PBRTQC and Statistical IQC
Table 2 Regulatory Authorities Governing IVD Quality Controls, by Country/Region
Table 3 IVD Quality Control Product Pricing
Table 4 Global IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 5 Global Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 6 Global Quality Control Products Market, by Country/Region, 2022–2031 (USD Million)
Table 7 Global Serum/Plasma-Based Controls Market, by Country/Region, 2022–2031 (USD Million)
Table 8 Global Whole Blood-Based Controls Market, by Country/Region, 2022–2031 (USD Million)
Table 9 Global Urine-Based Controls Market, by Country/Region, 2022–2031 (USD Million)
Table 10 Global Other Controls Market, by Country/Region, 2022–2031 (USD Million)
Table 11 Global Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 12 Global Independent Controls Market, by Country/Region, 2022–2031 (USD Million)
Table 13 Global Instrument-Specific Controls Market, by Country/Region, 2022–2031 (USD Million)
Table 14 Global Quality Assessment Services Market, by Country/Region, 2022–2031 (USD Million)
Table 15 Global Data Management Solutions Market, by Country/Region, 2022–2031 (USD Million)
Table 16 Global IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 17 Global IVD Quality Control Market for Immunoassay/Immunochemistry, by Country/Region, 2022–2031 (USD Million)
Table 18 Global IVD Quality Control Market for Biochemistry/Clinical Chemistry, by Country/Region, 2022–2031 (USD Million)
Table 19 Global IVD Quality Control Market for Molecular Diagnostics, by Country/Region, 2022–2031 (USD Million)
Table 20 Global IVD Quality Control Market for Hematology, by Country/Region, 2022–2031 (USD Million)
Table 21 Global IVD Quality Control Market for Coagulation/Hemostasis, by Country/Region, 2022–2031 (USD Million)
Table 22 Global IVD Quality Control Market for Microbiology, by Country/Region, 2022–2031 (USD Million)
Table 23 Global IVD Quality Control Market for Other Technologies, by Country/Region, 2022–2031 (USD Million)
Table 24 Global IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 25 Global IVD Quality Control Market for Infectious Diseases, by Country/Region, 2022–2031 (USD Million)
Table 26 Increase in the Number of New Cancer Cases Globally (2020-2040)
Table 27 Global IVD Quality Control Market for Oncology, by Country/Region, 2022–2031 (USD Million)
Table 28 Global IVD Quality Control Market for Cardiology, by Country/Region, 2022–2031 (USD Million)
Table 29 Global IVD Quality Control Market for Autoimmune Disorders, by Country/Region, 2022–2031 (USD Million)
Table 30 Global IVD Quality Control Market for Neurology, by Country/Region, 2022–2031 (USD Million)
Table 31 Global IVD Quality Control Market for Other Applications, by Country/Region, 2022–2031 (USD Million)
Table 32 Global IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 33 Global IVD Quality Control Market for Hospitals & Clinics, by Country/Region, 2022–2031 (USD Million)
Table 34 Global IVD Quality Control Market for Diagnostic Laboratories, by Country/Region, 2022–2031 (USD Million)
Table 35 Global IVD Quality Control Market for Academic Institutes & Research Laboratories, by Country/Region, 2022–2031 (USD Million)
Table 36 Global IVD Quality Control Market for Other End Users, by Country/Region, 2022–2031 (USD Million)
Table 37 Global IVD Quality Control Market, by Country/Region, 2022–2031 (USD Million)
Table 38 North America: Estimated Cancer Cases, 2020–2030
Table 39 North America: IVD Quality Control Market, by Country, 2022–2031 (USD Million)
Table 40 North America: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 41 North America: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 42 North America: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 43 North America: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 44 North America: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 45 North America: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 46 U.S.: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 47 U.S.: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 48 U.S.: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 49 U.S.: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 50 U.S.: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 51 U.S.: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 52 Canada: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 53 Canada: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 54 Canada: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 55 Canada: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 56 Canada: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 57 Canada: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 58 Europe: IVD Quality Control Market, by Country/Region, 2022–2031 (USD Million)
Table 59 Europe: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 60 Europe: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 61 Europe: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 62 Europe: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 63 Europe: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 64 Europe: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 65 Germany: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 66 Germany: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 67 Germany: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 68 Germany: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 69 Germany: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 70 Germany: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 71 France: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 72 France: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 73 France: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 74 France: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 75 France: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 76 France: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 77 U.K.: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 78 U.K.: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 79 U.K.: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 80 U.K.: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 81 U.K.: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 82 U.K.: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 83 Italy: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 84 Italy: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 85 Italy: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 86 Italy: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 87 Italy: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 88 Italy: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 89 Spain: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 90 Spain: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 91 Spain: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 92 Spain: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 93 Spain: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 94 Spain: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 95 Rest of Europe: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 96 Rest of Europe: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 97 Rest of Europe: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 98 Rest of Europe: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 99 Rest of Europe: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 100 Rest of Europe: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 101 Asia-Pacific: IVD Quality Control Market, by Country/Region, 2022–2031 (USD Million)
Table 102 Asia-Pacific: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 103 Asia-Pacific: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 104 Asia-Pacific: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 105 Asia-Pacific: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 106 Asia-Pacific: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 107 Asia-Pacific: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 108 China: Mass Testing Drives to Control the Spread of Covid-19 Infections
Table 109 China: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 110 China: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 111 China: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 112 China: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 113 China: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 114 China: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 115 Japan: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 116 Japan: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 117 Japan: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 118 Japan: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 119 Japan: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 120 Japan: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 121 India: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 122 India: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 123 India: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 124 India: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 125 India: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 126 India: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 127 Rest of Asia-Pacific: Estimated Number of New Cancer Cases, by Country (2020–2030)
Table 128 Rest of Asia-Pacific: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 129 Rest of Asia-Pacific: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 130 Rest of Asia-Pacific: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 131 Rest of Asia-Pacific: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 132 Rest of Asia-Pacific: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 133 Rest of Asia-Pacific: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 134 Latin America: IVD Quality Control Market, by Country/Region, 2022–2031 (USD Million)
Table 135 Latin America: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 136 Latin America: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 137 Latin America: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 138 Latin America: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 139 Latin America: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 140 Latin America: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 141 Brazil: Testing Initiatives for COVID-19
Table 142 Brazil: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 143 Brazil: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 144 Brazil: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 145 Brazil: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 146 Brazil: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 147 Brazil: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 148 Mexico: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 149 Mexico: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 150 Mexico: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 151 Mexico: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 152 Mexico: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 153 Mexico: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 154 Rest of Latin America: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 155 Rest of Latin America: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 156 Rest of Latin America: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 157 Rest of Latin America: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 158 Rest of Latin America: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 159 Rest of Latin America: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 160 Middle East & Africa: IVD Quality Control Market, by Offering, 2022–2031 (USD Million)
Table 161 Middle East & Africa: Quality Control Products Market, by Type, 2022–2031 (USD Million)
Table 162 Middle East & Africa: Quality Control Products Market, by Function, 2022–2031 (USD Million)
Table 163 Middle East & Africa: IVD Quality Control Market, by Technology, 2022–2031 (USD Million)
Table 164 Middle East & Africa: IVD Quality Control Market, by Application, 2022–2031 (USD Million)
Table 165 Middle East & Africa: IVD Quality Control Market, by End User, 2022–2031 (USD Million)
Table 166 Recent Developments, by Company, 2020–2024
List of Figures
Figure 1 Research Process
Figure 2 Secondary Sources Referenced for this Study
Figure 3 Primary Research Techniques
Figure 4 Key Executives Interviewed
Figure 5 Breakdown of Primary Interviews (Supply Side & Demand Side)
Figure 6 Market Sizing and Growth Forecast Approach
Figure 7 Global IVD Quality Control Market, by Offering, 2024 Vs. 2031 (USD Million)
Figure 8 Global IVD Quality Control Market, by Technology, 2024 Vs. 2031 (USD Million)
Figure 9 Global IVD Quality Control Market, by Application, 2024 Vs. 2031 (USD Million)
Figure 10 Global IVD Quality Control Market, by End User, 2024 Vs. 2031 (USD Million)
Figure 11 Global IVD Quality Control Market, by Geography
Figure 12 Healthcare Expenditure as a Percentage of GDP in Major Countries, 2015 Vs. 2020
Figure 13 Estimated Number of People Living With HIV, by Region, 2015 Vs. 2021 (In Thousand)
Figure 14 Global Diabetes Prevalence in People Aged 20–79 Years, by Region, 2021 Vs. 2030 Vs. 2045 (In Million)
Figure 15 USFDA Regulatory Pathways for IVD Products
Figure 16 EU Regulatory Pathway - IVDR 2017/746
Figure 17 China: Medical Device Classification and Pre-Market Requirements for IVD Devices
Figure 18 Porter's Five Forces Analysis
Figure 19 Global In Vitro Diagnostics (IVD) Market, by Offering, 2024 Vs. 2031 (USD Million)
Figure 20 Global IVD Quality Control Market, by Offering, 2024 Vs. 2031 (USD Million)
Figure 21 Global IVD Quality Control Market, by Technology, 2024 Vs. 2031 (USD Million)
Figure 22 Global IVD Quality Control Market, by Application, 2024 Vs. 2031 (USD Million)
Figure 23 Global IVD Quality Control Market, by End User, 2024 Vs. 2031 (USD Million)
Figure 24 Global IVD Quality Control Market Assessment, by Country/Region, 2024–2031 (USD Million)
Figure 25 North America: IVD Quality Control Market Snapshot
Figure 26 Europe: IVD Quality Control Market Snapshot
Figure 27 Health Expenditure (2015–2020)
Figure 28 Asia-Pacific: IVD Quality Control Market Snapshot
Figure 29 China: Independent Medical Laboratories Market, 2015–2019 (USD Million)
Figure 30 Latin America: IVD Quality Control Market Snapshot
Figure 31 Key Growth Strategies Adopted by Leading Players, 2020–2024
Figure 32 IVD Quality Control Market: Competitive Benchmarking, by Offering
Figure 33 IVD Quality Control Market: Competitive Benchmarking, by Geography
Figure 34 Competitive Dashboard: IVD Quality Control Market
Figure 35 Siemens Healthineers AG: Financial Overview (2023)
Figure 36 Bio-Rad Laboratories, Inc.: Financial Overview (2023)
Figure 37 Danaher Corporation: Financial Overview (2023)
Figure 38 LGC GROUP: Financial Overview (2023)
Figure 39 Thermo Fisher Scientific Inc.: Financial Overview (2023)
Figure 40 QuidelOrtho Corporation: Financial Overview (2023)
Figure 41 Bio-Techne Corporation: Financial Overview (2023)

























Published Date: Jul-2024
Published Date: Jan-2024
Published Date: Jan-2024
Published Date: Sep-2022
Published Date: Sep-2022
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates