1. Overview
1.1. Market Definition & Scope
1.2. Market Ecosystem
1.3. Currency and Limitations
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Approach
2.2. Process of Data Collection & Validation
2.2.1. Secondary Research
2.2.2. Primary Research/Interviews with Key Opinion Leaders from the Industry
2.3. Market Sizing and Forecast
2.3.1. Market Size Estimation Approach
2.3.2. Growth Forecast Approach
2.3.3. Market Share Analysis
2.4. Assumptions For the Study
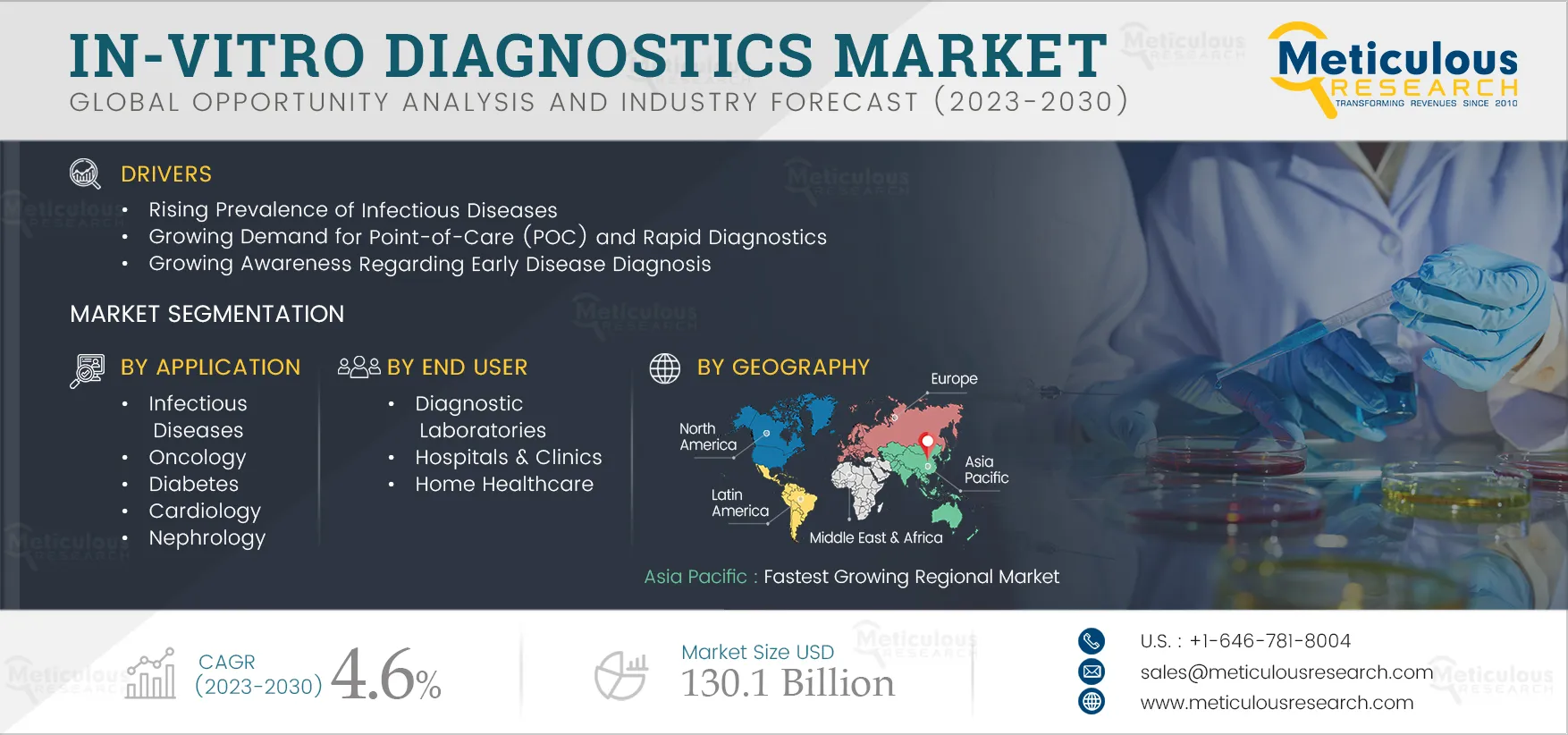
3. Executive Summary
4. Market Insights
4.1. Market Overview
4.2. Drivers
4.2.1. Rising Prevalence of Chronic Diseases Coupled with Increasing Geriatric Population
4.2.2. Increasing Incidence of Infectious Diseases
4.2.3. Growing Demand for Point-of-Care (POC) and Rapid Diagnostics
4.2.4. Rising Awareness Regarding Early Disease Diagnosis
4.2.5. Growing Healthcare Expenditure
4.2.6. Increasing Funding for Research Activities
4.3. Restraints
4.3.1. Stringent Technical Requirements and Regulatory Processes for High/Moderate-complexity Tests
4.3.2. Variance in Test Results Observed in Rapid IVD Tests
4.4. Opportunities
4.4.1. Emerging Economies
4.4.2. Inclination Toward Personalized Medicine
4.4.3. Advancements in Genomics and Proteomics
4.5. Challenges
4.5.1. Evolving Regulatory Landscape
4.6. Technology Trends
4.6.1. Integration of Artificial Intelligence & Machine Learning in Molecular Diagnostics
4.6.2. Development of Smartphone-based Detection Platforms for Rapid Diagnostic Tests
4.6.3. Increasing Applications of Next-generation Sequencing Technology
4.7. Macroindicators
4.7.1. Prevalence of Infectious and Chronic Diseases
4.7.2. Number of HIV Cases
4.7.3. Number of New Cancer Cases
4.7.4. Healthcare Expenditure as a Percentage of GDP, by Country
4.7.5. Biopharmaceutical R&D Expenditure
4.7.6. Percentage of Urban Population in Emerging Countries
4.8. Regulatory Analysis
4.8.1. North America
4.8.1.1. U.S.
4.8.1.2. Canada
4.8.2. Europe
4.8.3. Asia-Pacific
4.8.3.1. China
4.8.3.2. Japan
4.8.3.3. India
4.8.4. Latin America
4.8.5. Middle East
4.9. Value Chain Analysis
4.10. Technology Evolution/Market Evolution
4.11. Patent Analysis
4.12. Porter’s Five Forces Analysis
4.13. PESTLE Analysis
4.14. Consumer Behavior Analysis
4.14.1. Vendor Selection Criteria
4.14.2. Unmet Needs
4.14.3. Vendor/Brand Analysis
4.14.4. Product/Technology Adoption Trends
5. In Vitro Diagnostics (IVD) Market Assessment—by Offering
5.1. Overview
5.2. Reagents & Kits
5.3. Systems
5.4. Software & Services
6. In Vitro Diagnostics (IVD) Market Assessment—by Technology
6.1. Overview
6.2. Molecular Diagnostics
6.2.1. Polymerase Chain Reaction (PCR)
6.2.2. Hybridization
6.2.3. Isothermal Nucleic Acid Amplification Technology
6.2.4. DNA Sequencing & Next-generation Sequencing
6.2.5. Microarray
6.2.6. Mass Spectrometry
6.2.7. Other Molecular Diagnostic Technologies
6.3. Point-of-Care (POC) Diagnostics
6.3.1. Lateral Flow Assays/Rapid Tests
6.3.2. POC Molecular Diagnostics
6.3.3. Other PoC Products
6.4. Immunoassay/Immunochemistry
6.4.1. Enzyme-linked Immunosorbent Assays (ELISA)
6.4.2. Enzyme-linked Immunospot Assays
6.4.3. Western Blotting
6.4.4. Radioimmunoassay
6.5. Biochemistry/Clinical Chemistry
6.5.1. Metabolic Panels
6.5.2. Electrolyte Panels
6.5.3. Liver Panels
6.5.4. Lipid Profiles
6.5.5. Renal Profiles
6.5.6. Thyroid Function Panels
6.6. Whole Blood Glucose Monitoring
6.7. Microbiology
6.8. Hematology
6.9. Coagulation/Hemostasis
6.10. Urinalysis
6.11. Other IVD Technologies
7. In Vitro Diagnostics (IVD) Market Assessment—by Application
7.1. Overview
7.2. Infectious Diseases
7.2.1. Sexually Transmitted Diseases (STD) Testing
7.2.2. Healthcare-associated Infections (HAIs)
7.2.3. Hepatitis
7.2.4. HIV
7.2.5. Tropical Diseases
7.2.6. Influenza
7.2.7. Respiratory Infections (Excluding Influenza)
7.2.8. Other Infectious Diseases
7.3. Oncology
7.4. Diabetes
7.5. Cardiology
7.6. Nephrology
7.7. Autoimmune Disorders
7.8. Other Applications
8. In Vitro Diagnostics (IVD) Market Assessment—by Diagnostic Approach
8.1. Overview
8.2. Lab Testing
8.3. OTC/Self Testing
8.4. Point-of-Care Testing
9. In Vitro Diagnostics (IVD) Market Assessment—by End User
9.1. Overview
9.2. Diagnostic Laboratories
9.3. Hospitals & Clinics
9.4. Home Healthcare
9.5. Other End Users
10. In Vitro Diagnostics (IVD) Market Assessment—by Geography
10.1. Overview
10.2. North America
10.2.1. U.S.
10.2.2. Canada
10.3. Europe
10.3.1. Germany
10.3.2. France
10.3.3. U.K.
10.3.4. Italy
10.3.5. Spain
10.3.6. Switzerland
10.3.7. Rest of Europe
10.4. Asia Pacific
10.4.1. Japan
10.4.2. China
10.4.3. India
10.4.4. Australia
10.4.5. South Korea
10.4.6. Rest of Asia Pacific
10.5. Latin America
10.5.1. Brazil
10.5.2. Mexico
10.5.3. Argentina
10.5.4. Rest of Latin America
10.6. Middle East
10.6.1. Saudi Arabia
10.6.2. Rest of Middle East
10.7. Africa
10.7.1. South Africa
10.7.2. Rest of Africa
11. Competition Analysis
11.1. Overview
11.2. Key Growth Strategies
11.3. Competitive Dashboard
11.3.1. Industry Leaders
11.3.2. Market Differentiators
11.3.3. Vanguards
11.3.4. Emerging Companies
11.4. Competitive Benchmarking
11.5. List of Key Distributors
11.6. Product Pipeline Analysis
11.7. Market Share Analysis, By Company (2023)
11.7.1. F. Hoffmann-La Roche Ltd.
11.7.2. Abbott Laboratories
11.7.3. Danaher Corporation
11.7.4. Siemens Healthineers AG
11.7.5. Thermo Fisher Scientific Inc.
12. Company Profiles (Business Overview, Financial Overview, Product Portfolio, Strategic Developments, and SWOT Analysis*)
12.1. Abbott Laboratories
12.2. Agilent Technologies, Inc.
12.3. Becton, Dickinson and Company
12.4. bioMérieux SA
12.5. Bio-Rad Laboratories, Inc.
12.6. Danaher Corporation
12.7. DiaSorin S.p.A.
12.8. F. Hoffmann-La Roche Ltd.
12.9. Illumina, Inc.
12.10. QuidelOrtho Corporation
12.11. QIAGEN N.V.
12.12. Shenzhen Mindray Bio-Medical Electronics Co., Ltd
12.13. Siemens Healthineers AG
12.14. Thermo Fisher Scientific Inc.
12.15. Wama Diagnostica
12.16. Wiener Laboratorios SAIC
12.17. Hologic, Inc.
12.18. Abcam Plc.
12.19. Raybiotech, Inc.
12.20. Oy Medix Biochemica Ab
12.21. InBios International, Inc.
12.22. Dextra Laboratories Ltd.
12.23. GeneCopoeia, Inc.
12.24. Sakura Finetek USA, Inc.
12.25. Edvotek, Inc.
(Note: SWOT Analysis of the Top 5 Companies Will Be Provided)
13. Appendix
13.1. Available Customization
13.2. Related Reports
List of Tables
Table 1 Number of People with Diabetes Aged 20–79, by Region, 2021 Vs. 2030 Vs. 2045 (Thousand)
Table 2 Population Aged 65 Years or Above, by Region, 2019 Vs. 2030 Vs. 2050 (Million)
Table 3 Estimated Number of New Cancer Cases, by Type (2020 Vs. 2030)
Table 4 HIV Statistics (2021)
Table 5 Healthcare Expenditure as a Percentage of GDP, by Country (2014 Vs. 2019)
Table 6 Healthcare Expenditure, by Country (2020–2022)
Table 7 Requirements For CLIA-Waived and Moderate/High-complexity POC Molecular Diagnostic Tests
Table 8 Emerging Countries: Percentage of Urban Population (2021 Vs. 2025 Vs. 2050)
Table 9 Biomarkers and Corresponding Targeted Therapies Currently Available
Table 10 Clinical Applications of Genomics and Proteomics in Infectious Disease Diagnosis
Table 11 Regulatory Authorities Governing In Vitro Diagnostics, by Country
Table 12 Prevalence of Infectious and Chronic Diseases
Table 13 HIV Statistics
Table 14 Estimated Number of New Cancer Cases (2020 Vs. 2030)
Table 15 Healthcare Expenditures as a Percentage of GDP, by Country
Table 16 Biopharmaceutical R&D Expenditure, 2016–2028 (USD Billion)
Table 17 Percentage of Urban Population in Emerging Countries (2021 Vs. 2025 Vs. 2050)
Table 18 Some of The Emergency Use Authorizations (EUAs) Granted for IVD During the COVID-19 Pandemic
Table 19 Global In Vitro Diagnostics (IVD) Market, by Offering, 2022–2031 (USD Million)
Table 20 Global IVD Kits & Reagents Market, by Country/Region, 2022–2031 (USD Million)
Table 21 Global IVD Systems Market, by Country/Region, 2022–2031 (USD Million)
Table 22 Global IVD Software & Services Market, by Country/Region, 2022–2031 (USD Million)
Table 23 Global In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 24 Global Molecular Diagnostics Market, by Type, 2022–2031 (USD Million)
Table 25 Global Molecular Diagnostics Market, by Country/Region, 2022–2031 (USD Million)
Table 26 Key Companies Offering PCR Products
Table 27 Global Polymerase Chain Reaction Market, by Country/Region, 2022–2031 (USD Million)
Table 28 Global Hybridization Market, by Country/Region, 2022–2031 (USD Million)
Table 29 Key Companies Offering Isothermal Nucleic Acid Amplification Products
Table 30 Global Isothermal Nucleic Acid Amplification Market, by Country/Region, 2022–2031 (USD Million)
Table 31 Key Companies Offering DNA Sequencing & Next-Generation Sequencing Products
Table 32 Global DNA Sequencing and Next-Generation Sequencing Market, by Country/Region, 2022–2031 (USD Million)
Table 33 Key Companies Offering Microarray Products
Table 34 Global Microarrays Market, by Country/Region, 2022–2031 (USD Million)
Table 35 Global Mass Spectrometry Market, by Country/Region, 2022–2031 (USD Million)
Table 36 Other Molecular Diagnostic Technologies Market, by Country/Region, 2022–2031 (USD Million)
Table 37 Global Point-of-Care (POC) Diagnostics Market, by Technology, 2022–2031, (USD Million)
Table 38 Global Point-of-Care (POC) Diagnostics Market, by Country/Region, 2022–2031, (USD Million)
Table 39 Global Lateral Flow Assays/Rapid Tests Market, by 2022–2031 (USD Million)
Table 40 Global POC Molecular Diagnostics Market, 2022–2031 (USD Million)
Table 41 Other POC Products Market, by Country/Region, 2022–2031 (USD Million)
Table 42 Global Immunoassay/Immunochemistry Market, by Type, 2022–2031 (USD Million)
Table 43 Global Immunoassay/Immunochemistry Market, by Country/Region, 2022–2031 (USD Million)
Table 44 Global Enzyme-linked Immunosorbent Assays (ELISA) Market, by Country/Region, 2022–2031 (USD Million)
Table 45 EliSpot Vs. Conventional ELISA
Table 46 Global Enzyme-linked ImmunoSpot Assays (Elispot) Market, by Country/Region, 2022–2031 (USD Million)
Table 47 Global Western Blotting Market, by Country/Region, 2022–2031 (USD Million)
Table 48 Global Radioimmunoassay Market, by Country/Region, 2022–2031 (USD Million)
Table 49 Global Biochemistry/Clinical Chemistry Market, by Type, 2022–2031 (USD Million)
Table 50 Global Metabolic Panels Market, by Country/Region, 2022–2031 (USD Million)
Table 51 Global Electrolyte Panels Market, by Country/Region, 2022–2031, (USD Million)
Table 52 Global Liver Panels Market, by Country/Region, 2022–2031, (USD Million)
Table 53 Global Lipid Profiles Market, by Country/Region, 2022–2031 (USD Million)
Table 54 Global Renal Profiles Market, by Country/Region, 2022–2031 (USD Million)
Table 55 Global Thyroid Function Panels Market, by Country/Region, 2022–2031 (USD Million)
Table 56 Number of People with Diabetes Aged 20–79 Years, by Region, 2019 Vs. 2030 Vs. 2045 (In Million)
Table 57 Global Whole Blood Glucose Monitoring Market, by Country/Region, 2022–2031 (USD Million)
Table 58 Key Companies Offering Microbiology-based IVD Products
Table 59 Global Microbiology Market, by Country/Region, 2022–2031 (USD Million)
Table 60 Key Companies Offering Hematology Products
Table 61 Global Hematology Market, by Country/Region, 2022–2031 (USD Million)
Table 62 Key Companies Offering Coagulation and Hemostasis Products
Table 63 Global Coagulation and Hemostasis Market, by Country/Region, 2022–2031 (USD Million)
Table 64 Key Companies Offering Urinalysis Products
Table 65 Global Urinalysis Market, by Country/Region, 2022–2031 (USD Million)
Table 66 Global Other IVD Technologies Market, by Country/Region, 2022–2031 (USD Million)
Table 67 Global In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 68 Global In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 69 Global In Vitro Diagnostics Market for Infectious Diseases, by Country/Region, 2022–2031 (USD Million)
Table 70 Recently Launched and Approved Rapid Diagnostic Tests for COVID-19 Detection
Table 71 Global In Vitro Diagnostics Market for COVID-19 Testing, by Country/Region, 2022–2031 (USD Million)
Table 72 Global In Vitro Diagnostics Market for Sexually Transmitted Diseases (STD) Testing, by Country/Region, 2022–2031 (USD Million)
Table 73 Global Prevalence Of HIV, by Region (2022)
Table 74 Global In Vitro Diagnostics Market For HIV, by Country/Region, 2022–2031 (USD Million)
Table 75 Global In Vitro Diagnostics Market for Healthcare Acquired Infections (HAIs), by Country/Region, 2022–2031 (USD Million)
Table 76 Global In Vitro Diagnostics Market for Hepatitis, by Country/Region, 2022–2031 (USD Million)
Table 77 Global In Vitro Diagnostics Market For HIV, by Country/Region, 2022–2031 (USD Million)
Table 78 Global In Vitro Diagnostics Market for Tropical Diseases, by Country/Region, 2022–2031 (USD Million)
Table 79 Global In Vitro Diagnostics Market for Influenza, by Country/Region, 2022–2031 (USD Million)
Table 80 Global In Vitro Diagnostics Market for Respiratory Infections, by Country/Region, 2022–2031 (USD Million)
Table 81 Global In Vitro Diagnostics Market for Other Infectious Diseases, by Country/Region, 2022–2031 (USD Million)
Table 82 Global In Vitro Diagnostics Market for Oncology, by Country/Region, 2022–2031 (USD Million)
Table 83 Number of People with Diabetes Aged 20–79 Years, by Region, 2021 Vs. 2030 Vs. 2045 (In Million)
Table 84 Global In Vitro Diagnostics Market for Diabetes, by Country/Region, 2022–2031 (USD Million)
Table 85 Global In Vitro Diagnostics Market for Cardiology, by Country/Region, 2022–2031 (USD Million)
Table 86 Global In Vitro Diagnostics Market for Nephrology, by Country/Region, 2022–2031 (USD Million)
Table 87 Global In Vitro Diagnostics Market for Autoimmune Disorders, by Country/Region, 2022–2031 (USD Million)
Table 88 Global In Vitro Diagnostics Market for Other Applications, by Country/Region, 2022–2031 (USD Million)
Table 89 Global In Vitro Diagnostics Market, by Diagnostic Approach 2022–2031 (USD Million)
Table 90 Global In Vitro Diagnostics Market for Laboratory Testing, by Country/Region, 2022–2031 (USD Million)
Table 91 Global In Vitro Diagnostics Market For OTC/Self-Testing, by Country/Region, 2022–2031 (USD Million)
Table 92 Global In Vitro Diagnostics Market for Point-of-Care Testing, by Country/Region, 2022–2031 (USD Million)
Table 93 Global In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 94 Global In Vitro Diagnostics Market for Hospitals & Clinics, by Country/Region, 2022–2031 (USD Million)
Table 95 Global In Vitro Diagnostics Market for Diagnostic Laboratories, by Country/Region, 2022–2031 (USD Million)
Table 96 Global In Vitro Diagnostics Market for Home Healthcare, by Country/Region, 2022–2031 (USD Million)
Table 97 Global In Vitro Diagnostics Market for Other End Users, by Country/Region, 2022–2031 (USD Million)
Table 98 Global In Vitro Diagnostics Market, by Country/Region, 2022 Vs. 2031 (USD Million)
Table 99 North America: In Vitro Diagnostics Market, by Country, 2022–2031 (USD Million)
Table 100 North America: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 101 North America: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 102 North America: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 103 North America: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 104 North America: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 105 North America: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 106 North America: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 107 North America: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 108 North America: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 109 North America: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 110 U.S.: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 111 U.S.: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 112 U.S.: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 113 U.S.: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 114 U.S.: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 115 U.S.: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 116 U.S.: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 117 U.S.: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 118 U.S.: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 119 U.S.: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 120 Canada: Prevalence of Diseases/Conditions in The Geriatric (Age 65 Years and Above) Population (2019–2020)
Table 121 Canada: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 122 Canada: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 123 Canada: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 124 Canada: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 125 Canada: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 126 Canada: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 127 Canada: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 128 Canada: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 129 Canada: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 130 Canada: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 131 Europe: In Vitro Diagnostics Market, by Country/Region, 2022–2031 (USD Million)
Table 132 Europe: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 133 Europe: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 134 Europe: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 135 Europe: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 136 Europe: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 137 Europe: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 138 Europe: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 139 Europe: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 140 Europe: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 141 Europe: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 142 Germany: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 143 Germany: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 144 Germany: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 145 Germany: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 146 Germany: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 147 Germany: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 148 Germany: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 149 Germany: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 150 Germany: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 151 Germany: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 152 France: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 153 France: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 154 France: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 155 France: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 156 France: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 157 France: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 158 France: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 159 France: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 160 France: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 161 France: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 162 U.K.: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 163 U.K.: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 164 U.K.: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 165 U.K.: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 166 U.K.: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 167 U.K.: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 168 U.K.: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 169 U.K.: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 170 U.K.: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 171 U.K.: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 172 Italy: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 173 Italy: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 174 Italy: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 175 Italy: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 176 Italy: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 177 Italy: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 178 Italy: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 179 Italy: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 180 Italy: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 181 Italy: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 182 Spain: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 183 Spain: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 184 Spain: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 185 Spain: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 186 Spain: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 187 Spain: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 188 Spain: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 189 Spain: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 190 Spain: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 191 Spain: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 192 Switzerland: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 193 Switzerland: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 194 Switzerland: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 195 Switzerland: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 196 Switzerland: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 197 Switzerland: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 198 Switzerland: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 199 Switzerland: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 200 Switzerland: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 201 Switzerland: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 202 Rest of Europe: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 203 Rest of Europe: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 204 Rest of Europe: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 205 Rest of Europe: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 206 Rest of Europe: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 207 Rest of Europe: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 208 Rest of Europe: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 209 Rest of Europe: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 210 Rest of Europe: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 211 Rest of Europe: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 212 Asia-Pacific: In Vitro Diagnostics Market, by Country/Region, 2022–2031 (USD Million)
Table 213 Asia-Pacific: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 214 Asia-Pacific: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 215 Asia-Pacific: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 216 Asia-Pacific: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 217 Asia-Pacific: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 218 Asia-Pacific: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 219 Asia-Pacific: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 220 Asia-Pacific: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 221 Asia-Pacific: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 222 Asia-Pacific: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 223 Japan: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 224 Japan: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 225 Japan: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 226 Japan: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 227 Japan: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 228 Japan: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 229 Japan: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 230 Japan: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 231 Japan: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 232 Japan: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 233 China: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 234 China: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 235 China: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 236 China: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 237 China: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 238 China: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 239 China: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 240 China: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 241 China: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 242 China: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 243 India: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 244 India: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 245 India: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 246 India: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 247 India: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 248 India: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 249 India: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 250 India: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 251 India: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 252 India: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 253 South Korea: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 254 South Korea: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 255 South Korea: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 256 South Korea: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 257 South Korea: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 258 South Korea: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 259 South Korea: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 260 South Korea: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 261 South Korea: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 262 South Korea: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 263 Australia: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 264 Australia: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 265 Australia: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 266 Australia: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 267 Australia: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 268 Australia: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 269 Australia: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 270 Australia: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 271 Australia: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 272 Australia: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 273 Rest of Asia-Pacific: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 274 Rest of Asia-Pacific: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 275 Rest of Asia-Pacific: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 276 Rest of Asia-Pacific: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 277 Rest of Asia-Pacific: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 278 Rest of Asia-Pacific: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 279 Rest of Asia-Pacific: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 280 Rest of Asia-Pacific: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 281 Rest of Asia-Pacific: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 282 Rest of Asia-Pacific: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 283 Latin America: In Vitro Diagnostics Market, by Country/Region, 2022–2031 (USD Million)
Table 284 Latin America: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 285 Latin America: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 286 Latin America: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 287 Latin America: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 288 Latin America: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 289 Latin America: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 290 Latin America: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 291 Latin America: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 292 Latin America: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 293 Latin America: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 294 Testing Initiatives for COVID-19 In Brazil
Table 295 Brazil: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 296 Brazil: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 297 Brazil: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 298 Brazil: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 299 Brazil: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 300 Brazil: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 301 Brazil: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 302 Brazil: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 303 Brazil: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 304 Brazil: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 305 Mexico: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 306 Mexico: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 307 Mexico: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 308 Mexico: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 309 Mexico: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 310 Mexico: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 311 Mexico: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 312 Mexico: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 313 Mexico: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 314 Mexico: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 315 Argentina: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 316 Argentina: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 317 Argentina: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 318 Argentina: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 319 Argentina: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 320 Argentina: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 321 Argentina: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 322 Argentina: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 323 Argentina: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 324 Argentina: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 325 Rest of Latin America: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 326 Rest of Latin America: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 327 Rest of Latin America: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 328 Rest of Latin America: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 329 Rest of Latin America: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 330 Rest of Latin America: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 331 Rest of Latin America: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 332 Rest of Latin America: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 333 Rest of Latin America: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 334 Rest of Latin America: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 335 Middle East: In Vitro Diagnostics Market, by Country/Region, 2022–2031 (USD Million)
Table 336 Middle East: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 337 Middle East: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 338 Middle East: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 339 Middle East: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 340 Middle East: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 341 Middle East: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 342 Middle East: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 343 Middle East: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 344 Middle East: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 345 Middle East: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 346 Saudi Arabia: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 347 Saudi Arabia: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 348 Saudi Arabia: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 349 Saudi Arabia: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 350 Saudi Arabia: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 351 Saudi Arabia: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 352 Saudi Arabia: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 353 Saudi Arabia: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 354 Saudi Arabia: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 355 Saudi Arabia: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 356 Number of People Aged 65 Years and Above in The Middle East, 2017–2021 (In Thousands)
Table 357 Rest of Middle East: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 358 Rest of Middle East: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 359 Rest of Middle East: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 360 Rest of Middle East: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 361 Rest of Middle East: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 362 Rest of Middle East: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 363 Rest of Middle East: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 364 Rest of Middle East: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 365 Rest of Middle East: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 366 Rest of Middle East: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 367 Africa: In Vitro Diagnostics Market, by Country/Region, 2022–2031 (USD Million)
Table 368 Africa: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 369 Africa: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 370 Africa: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 371 Africa: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 372 Africa: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 373 Africa: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 374 Africa: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 375 Africa: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 376 Africa: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 377 Africa: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 378 South Africa: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 379 South Africa: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 380 South Africa: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 381 South Africa: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 382 South Africa: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 383 South Africa: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 384 South Africa: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 385 South Africa: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 386 South Africa: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 387 South Africa: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 388 Rest of Africa: In Vitro Diagnostics Market, by Offering, 2022–2031 (USD Million)
Table 389 Rest of Africa: In Vitro Diagnostics Market, by Technology, 2022–2031 (USD Million)
Table 390 Rest of Africa: In Vitro Diagnostics Market for Immunoassay/Immunochemistry, by Type, 2022–2031 (USD Million)
Table 391 Rest of Africa: In Vitro Diagnostics Market for Biochemistry/Clinical Chemistry, by Type, 2022–2031 (USD Million)
Table 392 Rest of Africa: In Vitro Diagnostics Market for Molecular Diagnostics, by Type, 2022–2031 (USD Million)
Table 393 Rest of Africa: In Vitro Diagnostics Market for Point-of-Care (POC) Diagnostics, by Type, 2022–2031 (USD Million)
Table 394 Rest of Africa: In Vitro Diagnostics Market, by Application, 2022–2031 (USD Million)
Table 395 Rest of Africa: In Vitro Diagnostics Market for Infectious Diseases, by Type, 2022–2031 (USD Million)
Table 396 Rest of Africa: In Vitro Diagnostics Market, by Diagnostics Approach, 2022–2031 (USD Million)
Table 397 Rest of Africa: In Vitro Diagnostics Market, by End User, 2022–2031 (USD Million)
Table 398 Recent Developments, by Company (2021–2024)
List of Figure
Figure 1 Research Process
Figure 2 Secondary Sources Referenced for this Study
Figure 3 Primary Research Techniques
Figure 4 Key Executives Interviewed
Figure 5 Breakdown of Primary Interviews (Supply-side & Demand-side)
Figure 6 Market Size Estimation
Figure 7 Global In Vitro Diagnostics Market, by Offering, 2024 Vs. 2031 (USD Million)
Figure 8 Global In Vitro Diagnostics Market, by Technology, 2024 Vs. 2031 (USD Million)
Figure 9 Global In Vitro Diagnostics Market, by Application, 2024 Vs. 2031 (USD Million)
Figure 10 Global In Vitro Diagnostics Market, by Diagnostic Approach, 2024 Vs. 2031 (USD Million)
Figure 11 Global In Vitro Diagnostics Market, by End User, 2024 Vs. 2031 (USD Million)
Figure 12 Global In Vitro Diagnostics Market, by Geography
Figure 13 Impact Analysis of Market Dynamics
Figure 14 U.S.: Medical and Health R&D Expenditure, 2018–2022 (USD Million)
Figure 15 Global Biopharmaceutical R&D Expenditure, 2018–2028 (USD Billion)
Figure 16 U.S. FDA Regulatory Pathways for IVD Kits
Figure 17 EU Regulatory Pathway - IVDR 2017/746
Figure 18 China: Medical Device Classification and Pre-Market Requirements for IVD Devices
Figure 19 Vendor Selection Criteria
Figure 20 Vendor/Brand Analysis
Figure 20 Global In Vitro Diagnostics Market, by Offering, 2024 Vs. 2031 (USD Million)
Figure 21 Global In Vitro Diagnostic Market, by Technology, 2024 Vs. 2031 (USD Million)
Figure 22 Global In Vitro Diagnostics Market, by Application, 2024 Vs. 2031 (USD Million)
Figure 23 U.S.: Estimated Acute Hepatitis C Cases, 2011–2020
Figure 24 Estimated Number of New Cancer Cases, by Geography, 2020–2040 (Million)
Figure 25 Total Number of Accredited Testing Laboratories (ISO/IEC 17025), 2016–2020
Figure 26 Prevalence of Diabetes Across the World (In Million)
Figure 27 Global In Vitro Diagnostics Market, by Diagnostic Approach 2024 Vs. 2031 (USD Million)
Figure 28 Benefits of Laboratory Testing Approach
Figure 29 Benefits of the Point-of-Care Testing Approach
Figure 30 Global In Vitro Diagnostics Market, by End User, 2024 Vs. 2031 (USD Million)
Figure 31 Global In Vitro Diagnostics Market, by Region, 2024 Vs. 2031 (USD Million)
Figure 32 North America: Number of People Aged 65 Years and Above, 2015–2021 (In Million)
Figure 33 North America: Per Capita Health Expenditure (USD), 2017–2021
Figure 34 Canada: Number of People Aged 65 And above, 2018–2022 (In Million)
Figure 35 Europe: Number of People Aged 65 And Above, In Millions (2015–2021)
Figure 36 Europe: In Vitro Diagnostics Market Snapshot
Figure 37 France: Percentage of Population Aged 65 Years and Above (2016–2021)
Figure 38 France: Prevalence of Chronic Diseases in People Aged 65 And Above (2020)
Figure 39 U.K: Number of Cancer Cases, 2010–2030 (In Million)
Figure 40 U.K.: Number of Diabetes and Urogenital, Blood, And Endocrine Disease Cases – DALYs (Disability-Adjusted Life Years), 2017–2020 (In Thousand)
Figure 41 U.K.: Number of People Diagnosed with Sexually Transmitted Infections, 2018–2020 (In Thousand)
Figure 42 Italy: Ovarian Cancer Cases Diagnosed (2018–2020)
Figure 43 Number of HIV Tests Performed in Catalonia (Spain) (2014–2021)
Figure 44 Switzerland: Number of New Cancer Cases Reported (2020–2040)
Figure 45 Switzerland: Number of People Aged 65 Years and Above (2017–2021)
Figure 46 Asia-Pacific: In Vitro Diagnostics Market Snapshot
Figure 47 Japan: Percentage Share of the Geriatric Population (Aged 65 Years and Above) (2015–2022)
Figure 48 Japan: New Cancer Cases Reported (2020–2040)
Figure 49 China: Independent Medical Laboratories Market, 2015–2021 (USD Million)
Figure 50 India: Number of People Aged 65 Years and Above, 2017–2021 (Million)
Figure 51 Latin America: In Vitro Diagnostics Market Snapshot
Figure 52 Brazil: Challenges to The In Vitro Diagnostics Market
Figure 53 Middle East: In Vitro Diagnostics Market Snapshot
Figure 54 Saudi Arabia: Number of People Aged 65 Years and Above, 2017–2021 (In Thousand)
Figure 55 Africa: In Vitro Diagnostics Market Snapshot
Figure 56 South Africa: Number of People (All Ages) Living With HIV, 2010–2020 (In Million)
Figure 57 South Africa: Number of People with Diabetes, 2000–2045 (In Thousand)
Figure 58 Key Growth Strategies Adopted by Leading Players (2021–2024)
Figure 59 Competitive Dashboard: Global In Vitro Diagnostics Market
Figure 60 Global In Vitro Diagnostics Market Competitive Benchmarking, by Offering
Figure 61 Competitive Dashboard In Vitro Diagnostics Market
Figure 62 Market Share Analysis: Global In Vitro Diagnostics Market (2023)
Figure 63 Abbott Laboratories: Financial Overview (2023)
Figure 64 Becton, Dickinson and Company: Financial Overview (2023)
Figure 65 bioMérieux SA: Financial Overview (2023)
Figure 66 Danaher Corporation: Financial Overview (2023)
Figure 67 F. Hoffman-La Roche Ltd: Financial Overview (2023)
Figure 68 QIAGEN N.V.: Financial Overview (2023)
Figure 69 Siemens Healthineers AG (A Subsidiary of Siemens Ag): Financial Overview (2023)
Figure 70 Thermo Fisher Scientific Inc.: Financial Overview (2023)
Figure 71 Bio-Rad Laboratories: Financial Overview (2023)
Figure 72 Illumina, Inc.: Financial Overview (2023)
Figure 73 Shenzhen Mindray Bio-Medical Electronics Co., Ltd: Financial Overview (2023)
Figure 74 QuidelOrtho Corporation; Financial Overview (2023)
Figure 75 Agilent Technologies, Inc.: Financial Overview (2023)
Figure 76 DiaSorin S.p.A: Financial Overview (2023)
Figure 77 Hologic, Inc.: Financial Overview (2023)
 Click here to:
Click here to: 






















