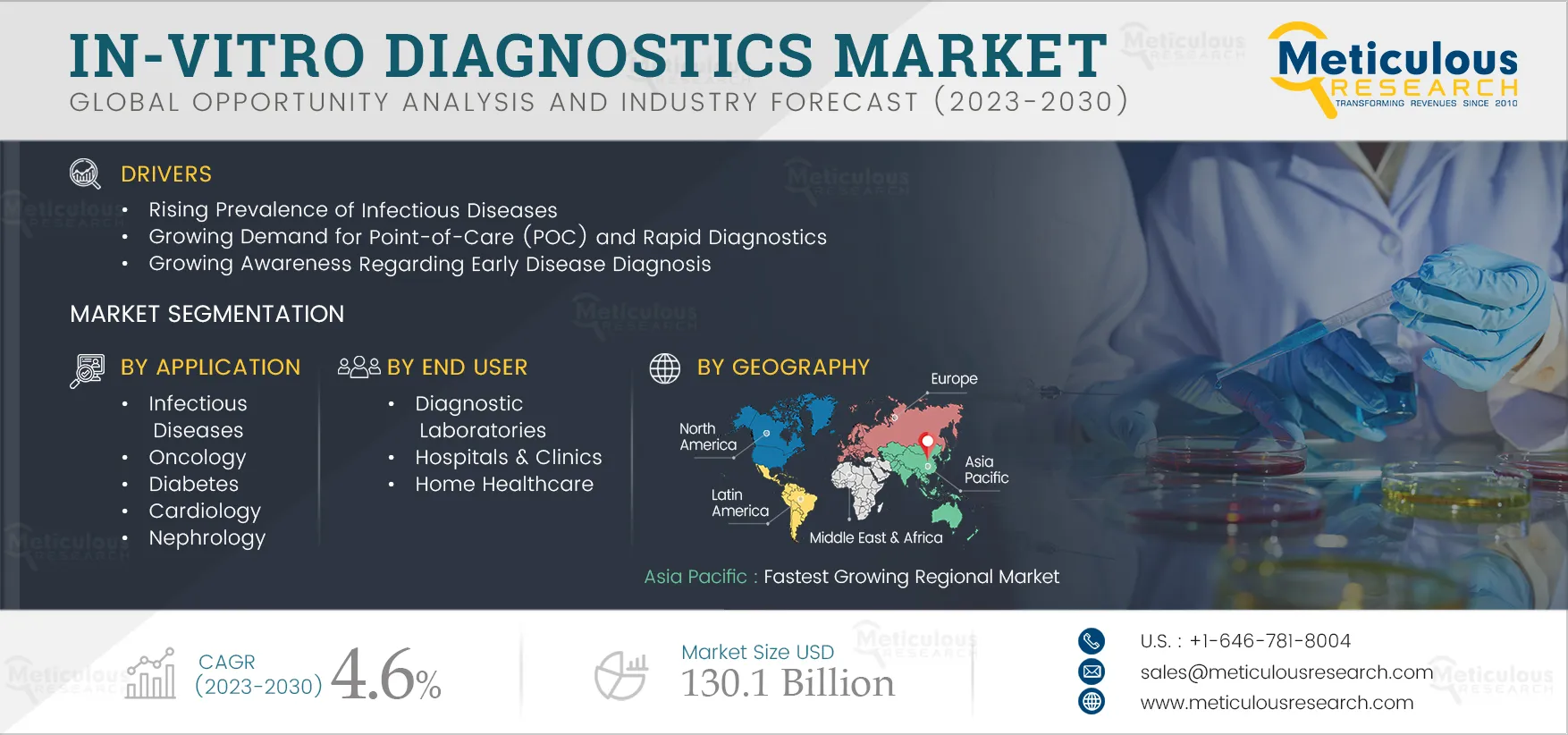
The In-vitro Diagnostics Market Will Register a CAGR of 5.3% During the Forecast Period to Reach $116.28 billion by 2031. IVD comprises consumables such as kits & reagents, instruments, software and services. The rising prevalence of chronic diseases coupled with the increasing geriatric population, rising prevalence of acute and chronic infectious diseases, increasing funding for research activities, growing awareness regarding early disease diagnosis, growing demand for point-of-care (POC) diagnostics and rapid diagnosis, rising healthcare expenditure, and increasing funding for research activities are driving the growth of the global IVD market.
Here are the top 10 companies operating in the In-vitro Diagnostics Market
F. Hoffmann-La Roche Ltd. (Switzerland)
Founded in 1896 and headquartered in Basel, Switzerland., F. Hoffmann-La Roche Ltd is a research-based healthcare company majorly engaged in creating innovative medicines and providing solutions for diagnostics. The company operates through two major business segments, namely, Pharmaceuticals and Diagnostics, and offers centralized and point-of-care kits & reagents, advanced staining reagents, molecular diagnostic tests, and blood screening tests through its diagnostics division. The Diagnostics segment is further categorized into Core Lab, Molecular Lab, Point of Care, Diabetes Care, and Pathology Lab. This segment manufactures equipment & reagents for research & medical diagnostic applications.
Hoffman-La Roche has 20 manufacturing sites and 27 research & development sites engaged in Pharmaceuticals and Diagnostics operations worldwide.
Abbott Laboratories (U.S.)
Founded in 1888 and headquartered in Illinois, U.S., Abbott Laboratories is engaged in the discovery, development, manufacturing, and sales of healthcare products. The company offers various products in diagnostics, medical devices, nutrition, and branded generic pharmaceuticals markets. Abbott operates worldwide through four business segments, namely, Established Pharmaceutical Products, Diagnostic Products, Nutritional Products, and Medical Devices.
The company operates in the in vitro diagnostics market through its Diagnostics business segment, which is further divided into Core Laboratory, Rapid Diagnostics, Point of Care, and Molecular business segments. The Rapid Diagnostics business is a part of Alere Inc. (U.S.), a diagnostic device manufacturer and service provider, which Abbott acquired in October 2017.
Abbott has a wide geographic presence and a strong distribution network through direct and indirect channels in various countries. The company has 90 manufacturing facilities globally, of which 24 manufacturing sites are engaged in developing diagnostic products.
Danaher Corporation (U.S.)
Established in 1984 and headquartered in Washington, D.C, U.S., Danaher Corporation is involved in the designing, manufacturing, and marketing of medical, professional, industrial, and commercial products & services. The company operates in three segments, namely, Life Sciences, Diagnostics, and Environmental & Applied Solutions.
The Diagnostics segment offers a wide range of in vitro diagnostics products, including analytical instruments, reagents, consumables, software, and services. These products are used in various healthcare settings such as hospitals, physicians’ offices, reference laboratories, and other critical care settings to diagnose diseases. This segment is further categorized into Clinical Lab Diagnostics, Critical Care Diagnostics, and Anatomical & Pathological Diagnostics.
Danaher Corporation has 212 R&D, manufacturing, sales, distribution, service, and administrative facilities in more than 60 countries worldwide. The company’s manufacturing facilities are located in North America, Europe, Asia, and Australia. Danaher Corporation has several subsidiaries across the globe, of which Beckman Coulter, Inc. (U.S.), Cepheid (U.S.), Leica Biosystems Nussloch GmbH (Germany), HemoCue AB (Sweden), and Radiometer Medical ApS (Denmark) offer instruments & reagents for in vitro diagnostic applications.
Siemens Healthineers AG (Germany)
Founded in 1847 and headquartered in Erlangen, Germany, Siemens Healthineers AG is engaged in developing and selling a wide range of products, including medical imaging applications, laboratory diagnostics, and point-of-care testing. The company also offers digital health platforms for various clinical specialties and laboratories across the globe.
Siemens Healthineers operates in four business segments, namely, Imaging, Diagnostics, Varian, and Advanced Therapies. The company operates in the in vitro diagnostics market through its Diagnostics segment. This segment offers in vitro diagnostic products & services to healthcare professionals in molecular diagnostics, point-of-care diagnostics, and laboratories.
Siemens has a direct presence in more than 70 countries, including the U.S., Canada, Germany, the U.K., France, Italy, Spain, Denmark, Sweden, Switzerland, Belgium, India, China, Japan, Australia, New Zealand, and the Republic of Korea.
Thermo Fisher Scientific Inc. (U.S.)
Founded in 1956 and headquartered in Massachusetts, U.S., Thermo Fisher Scientific Inc. is a biotechnology company engaged in life sciences research, improving patient diagnostics, and increasing laboratory productivity. The company operates through four business segments, namely, Life Sciences Solutions, Analytical Instruments, Specialty Diagnostics, and Laboratory Products and Biopharma Services. The company operates in the IVD market through its Specialty Diagnostics segment and offers diagnostic test kits, reagents, culture media, instruments, and associated products for customers in healthcare, clinical, pharmaceutical, industrial, and food safety laboratories.
Some of the major brands of Thermo Fisher are Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services, and Patheon. In June 2019, Thermo Fisher Scientific sold its Anatomical Pathology business to PHC Holdings Corporation (Japan), which primarily offered products for cancer diagnosis & medical research in histology, cytology, and hematology applications.
The company has a direct presence through its regional offices and production sites in the U.S., Mexico, Brazil, Germany, Belgium, Austria, the U.K., Italy, the Netherlands, South Africa, the UAE, India, China, Japan, Australia, and the Republic of Korea.
Becton, Dickinson and Company (BD)
Founded in 1887 and headquartered in New Jersey, U.S., Becton, Dickinson and Company (BD) is a medical technology company that manufactures and sells a wide range of medical devices, laboratory equipment, and diagnostic products used by healthcare institutions, clinical laboratories, pharmaceutical companies, and research centers. The company operates in three business segments, namely, BD Medical, BD Life Sciences, and BD Interventional.
The BD Life Sciences business segment offers products through two major categories, namely, Integrated Diagnostic Solutions (Diagnostic Systems and Preanalytical Systems) and Biosciences. The Diagnostic Systems category offers numerous products with clinical and industrial applications. These products include molecular testing systems for infectious diseases & women’s health, liquid-based cytology systems for cervical cancer screening, culturing systems, rapid diagnostic assays for testing respiratory infections, microorganism identification, and drug susceptibility systems.
BD has a direct presence in the U.S., Brazil, Canada, China, France, Spain, the U.K., Germany, Hungary, India, Ireland, Israel, Italy, Japan, Mexico, Singapore, and the Netherlands through its manufacturing and sales operations. The R&D facilities of the company are located in China, France, India, Ireland, the U.S., and Singapore. BD has a wide global distribution network and markets its products through independent distribution channels and directly to hospitals, healthcare institutions, and independent sales representatives.
bioMérieux SA (France)
Founded in 1963 and headquartered in Marcy-l’Étoile, France. bioMérieux is engaged in developing a wide range of products & solutions, including reagents, instruments, and software for clinical diagnostics. The company offers products for microbiology, immunoassay, molecular biology, and industrial applications.
bioMérieux operates through two reportable segments, namely, Clinical Applications and Industrial Applications. The Clinical Applications segment is further categorized into Molecular Biology, Microbiology, Immunoassays, and Other Ranges. The products offered through this segment are used to diagnose infectious diseases such as bacterial, parasitic, and viral infections.
The company has a direct presence and a strong distribution network across 160 countries through its 44 subsidiaries and 15 production sites. The activities carried out at these sites are the production, sales, and distribution of reagents for ready-to-use media for microbiology and industrial applications, research & development, and commercial and administrative functions.
Illumina, Inc. (U.S.)
Founded in 1998 and headquartered in California, U.S., Illumina, Inc., is a producer, marketer and developer of sequencing and array-based solutions for genomic analysis. The company operates its business under two segments, namely, Core Illumina and Grail. Illumina operates in the IVD market through its Core Illumina segment by providing NGS platforms for diagnostic use. The company offers its services to molecular diagnostic laboratories, government laboratories, academic institutions, hospitals, genomic research centers, biotechnology companies, consumer genomics companies, pharmaceuticals, and commercial companies.
The Core Illumina segment includes all the Illumina’s core operations. The company markets and distributes products directly to customers in North America, Latin America, Europe, and Asia-Pacific. Additionally, the company has a network of life-science distributors in certain markets within Europe, Asia-Pacific, Latin America, the Middle East, and Africa.
Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China)
Founded in 1991 and headquartered in Guangdong Sheng, China, Shenzhen Mindray Bio-Medical Electronics Co., Ltd provides a wide range of medical devices and IT ecosystem solutions. The company operates through five segments: Patient Monitoring & Life Support, In vitro Diagnostics, Medical Imaging, Other Products, and Others.
The In vitro Diagnostics segment of the company offers hematology analyzers, chemiluminescence immunoassay analyzers, biochemistry analyzers, coagulation analyzers, urine analyzers, microbiology diagnostic systems, and their related reagents, which are used in clinical diagnostics for samples such as blood, body fluids, and tissues.
The company has branches in more than 30 provinces and autonomous regions in China and subsidiaries in more than 30 countries globally. The company has 52 subsidiaries in North America, Europe, Asia, Africa, Latin America, and other regions, and its products are exported to more than 190 countries and regions.
Bio-Rad Laboratories, Inc. (U.S.)
Founded in 1952 and headquartered in California, U.S., Bio-Rad Laboratories, Inc. is a distributor and manufacturer of clinical diagnostics products and life science research. The company supplies and produces various systems and products for healthcare, analytical chemistry, and life sciences research. Bio-Rad Laboratories operates through two reportable segments: Clinical Diagnostics and Life Sciences. The company provides over 3,000 products through the Clinical Diagnostic segment, comprising over 300 clinical diagnostics tests, including IVD. The company’s offerings cater to physician offices, diagnostic reference laboratories, hospital laboratories, and transfusion laboratories.
The company has a direct distribution source in over 35 countries outside the U.S., including Afghanistan, France, Hong Kong, Japan, Korea, Iceland, Germany, Australia, Spain, the Netherlands, and South Africa.
Popular Mentions – QuidelOrtho Corporation (U.S.), Qiagen N.V. (Netherlands), Wama Diagnostica (Brazil), Weiner Laboratories SAIC (Argentina), DiaSorin S.p.A. (Italy), and Hologic, Inc. (U.S.).
 Click here to:
Click here to: 






















