Resources
About Us
IVD Assay Development Market Size, Share, Forecast, & Trends Analysis by Offering (Assay Development, Packaging Development) Technology (Immunoassay, Molecular Diagnostics, Biochemistry) Application (Oncology, Diabetes) – Global Forecast to 2031
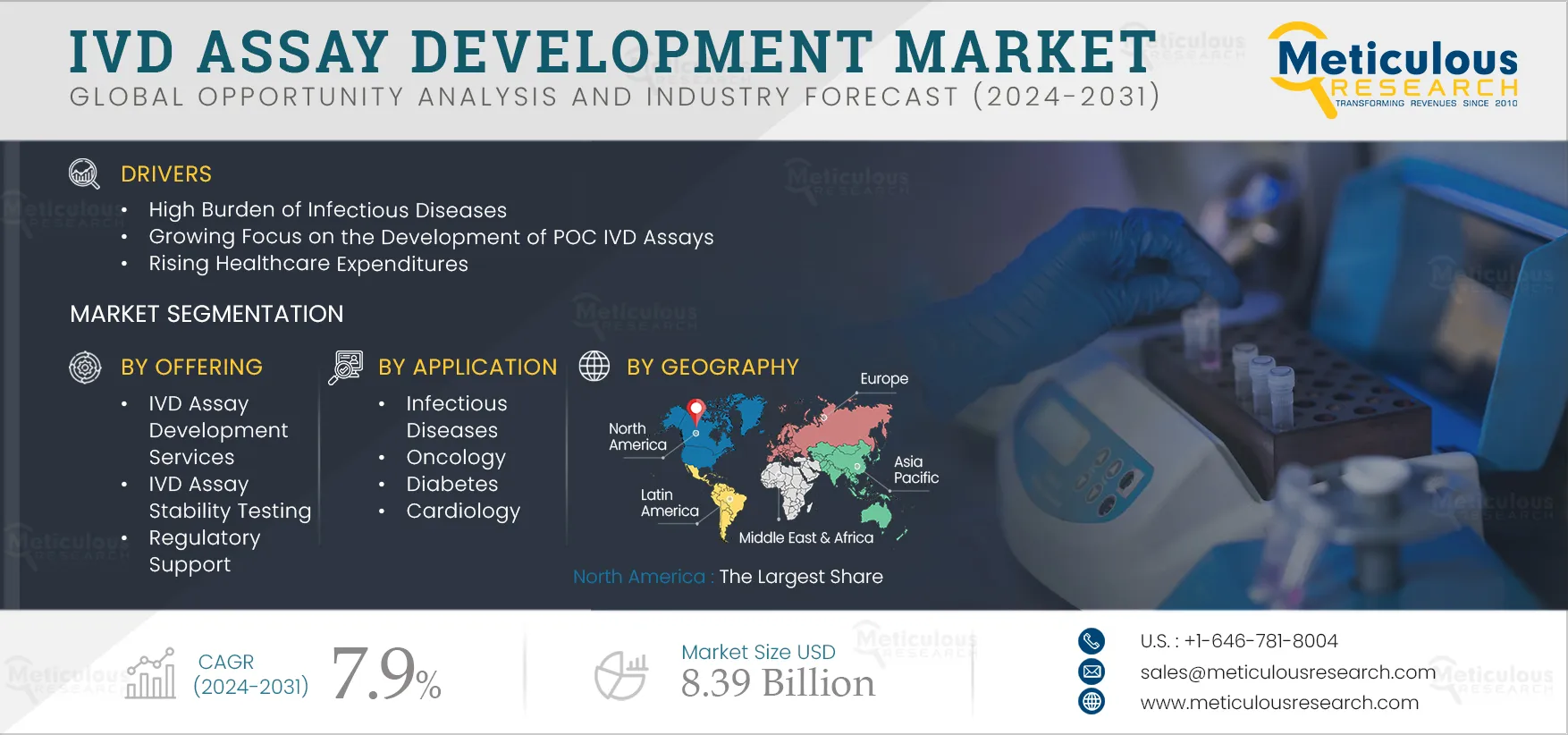
Report ID: MRHC - 1041354 Pages: 177 Oct-2024 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportKey factors driving the growth of this market include the rising prevalence of chronic diseases & the increasing geriatric population, the high burden of infectious diseases, the growing focus on the development of POC IVD assays, and rising healthcare expenditures. Furthermore, emerging economies and the use of advanced technologies in IVD assay development are expected to generate growth opportunities for the stakeholders in this market.
Key Findings
Click here to: Get Free Sample Pages of this Report
The prevalence of chronic diseases such as cancer, diabetes, arthritis, and heart disease is on the rise globally. According to the World Health Organization (WHO), chronic diseases account for 41 million deaths each year (around 74% of all deaths) globally. Growth in the geriatric population, tobacco & alcohol use, physical inactivity, and unhealthy diets are driving a steady increase in the prevalence of chronic diseases. According to the International Diabetes Federation (IDF), the global prevalence of diabetes is estimated to increase from 10.5% of the global population in 2021 to 11.3% in 2030. According to the IDF, in 2021, 537 million individuals were diagnosed with diabetes globally (Western Pacific accounted for 206 million cases, while Southeast Asia accounted for 90 million cases, the Middle East & North Africa for 73 million cases, Europe for 61 million cases, North America & the Caribbean for 51 million cases, South & Central America for 32 million cases, and Africa for 24 million cases). The number of diabetes cases is projected to reach 643 million in 2030 and 783 million in 2045.
Chronic diseases are often associated with the elderly population due to declining bodily functions and immunity in the population segment. Aging leads to progressive deterioration in the structure and functioning of organs, making the elderly population more prone to chronic diseases. According to World Population Prospects, the population aged 65 and over is growing more rapidly than other age groups. The percentage of the global population aged 65 years and over is expected to rise from 10% in 2022 to 16% in 2050. This remarkable growth in the elderly population is driven by declining fertility rates and improvements in longevity.
The WHO estimates that globally, 1 in 5 people develop cancer during their lifetime, and 1 in 8 men and 1 in 11 women die from the disease. As per WHO estimates, the global incidence of cancer is expected to increase from 19.2 million new cases in 2022 to 24.1 million new cases in 2030, while the global mortality from cancer is expected to increase from 9.7 million deaths in 2022 to 11.9 million deaths in 2030. This growing disease burden has increased the demand for IVD testing, resulting in IVD companies preferring to outsource the development of IVD assays to fulfill the growing demand for diagnostic assays while controlling production costs. These factors are driving the growth of the IVD assay development market.
Infectious diseases are caused by organisms such as bacteria, viruses, fungi, and parasites. Infectious diseases are transmitted from person to person or through bites from insects or animals. The burden of infectious diseases has increased globally, driving the need for safe and effective diagnostic assays. Hence, the demand for IVD assay development is expected to grow with the increasing utilization of diagnostic assays.
The following statistics indicate the high burden of infectious diseases:
Thus, the increasing burden of infectious diseases is expected to boost the need for diagnosis, driving the development of IVD assays.
IVD assays are developed using various technologies such as Polymerase Chain Reaction (PCR), Enzyme-linked Immunosorbent Assay (ELISA), Chemiluminescent Immunoassay (CLIA), Next-generation Sequencing (NGS), and proteomics. IVD assays are used to detect genomic variations in DNA. The use of genomic technologies has accelerated the shift toward precision medicine, wherein diagnostic tests and assays can be personalized to a person's genetic composition, enabling individualized treatment techniques based on each patient's unique genetic profile. Next-generation Sequencing (NGS) technology offers a comprehensive perspective of the human genome. Thus, it is used in IVD assay development to improve the accuracy of diagnosis, prognosis, and treatment selection.
In addition, High-throughput Sequencing (HTS) is used in the development of IVD assays for applications such as tumor profiling, HIV drug resistance detection, and early cancer detection. HTS provides multiple benefits, including retaining high precision while sequencing and sequencing millions of DNA molecules simultaneously. This can boost sequencing speed and provide more impactful conclusions by employing larger sample sizes.
Some of the recent key developments in this market are as follows:
Based on offering, the global IVD assay development market is segmented into IVD assay development services, IVD packaging development services, IVD assay stability testing, regulatory support, and other offerings. In 2024, the into IVD assay development services segment is estimated to account for the largest share of the global IVD assay development market. The large share of this segment is attributed to the trend of outsourcing IVD assay design and development among IVD companies, the high demand for IVD assays in drug discovery, increasing partnerships between manufacturers and research institutes to promote innovation and develop new assays, and the high prevalence of infectious diseases.
Assay development includes the designing of assays, proof of concept, feasibility studies with reagent formulation, prototype development, and complete assay development. The assay format is designed based on the analyte and the intended level of specificity and sensitivity. Many In Vitro Diagnostic (IVD) assays are developed using methods such as Enzyme-linked Immunosorbent Assay (ELISA), Polymerase Chain Reaction (PCR), lateral flow, cytology, Chemiluminescent Immunoassay (CLIA), and immunohistochemistry.
In addition, the rising use of IVD assays in drug discovery is driving the demand for IVD assay development services. IVD assays are critical in evaluating medication candidates, detecting biomarkers, guiding treatment decisions, and identifying patients for specific therapies. With pharmaceutical companies’ shift toward personalized medicine and precision therapeutics, the demand for high-quality IVD assays is growing rapidly.
However, the IVD assay stability testing segment is projected to register the highest CAGR during the forecast period of 2024 to 2031. IVD Stability testing assesses the consistency of IVD assays in various storage conditions, including humidity, temperature, and light exposure, to ensure consistent results. IVD assay stability testing improves product reliability and accuracy, increasing clients’ reliance on the product over time. These factors are expected to drive the segment’s growth during the forecast period.
In 2024, North America is estimated to account for the largest share of the global IVD assay development market. North America’s significant market share in the IVD assay development market is primarily attributed to the presence of leading IVD assay development companies in the region, the high prevalence of various infectious diseases and chronic diseases, the well-developed healthcare sector, awareness regarding early disease diagnosis, early adoption of advanced innovative diagnostic products, and significant research funding for the development of advanced diagnostic technologies.
However, the market in Asia-Pacific is projected to register the highest CAGR during the forecast period. This market's growth is attributed to factors like ongoing improvements in healthcare infrastructure and increasing government investment in the IVD sector. Accelerated economic growth in many Asia-Pacific countries has led governments to invest more in the healthcare sector to enhance accessibility to healthcare facilities and improve infrastructure. Additionally, growing awareness of health and treatment options, an expanding middle-class population with higher disposable income, increased health insurance penetration, and a rising aging population are further boosting the market’s growth in Asia-Pacific.
The report includes a competitive landscape based on an extensive assessment of the key growth strategies adopted by leading market players over the past three years (2021-2024).
The key players profiled in the global IVD assay development market report are Thermo Fisher Scientific Inc. (U.S.), Avioq, Inc. (U.S.), Creative Biolabs, Inc (U.S.), Maxim Biomedical, Inc. (U.S.), Bio-Techne Corporation (U.S.), Merck KGaA (Germany), PeploBio Ltd (U.K.), ICON plc (Ireland), NeoDx Biotech Labs Pvt. Ltd. (India), Eclevar Medtech (France), Savyon Diagnostics (Israel), Promega Corporation (U.S.), and Future Diagnostics Solutions B.V. (Netherlands).
|
Particulars |
Details |
|
Number of Pages |
177 |
|
Format |
|
|
Forecast Period |
2024–2031 |
|
Base Year |
2023 |
|
CAGR (Value) |
7.9% |
|
Market Size (Value) |
USD 8.39 Billion by 2031 |
|
Segments Covered |
By Offering
By Technology
By Application
|
|
Countries Covered |
North America (U.S., Canada), Europe (Germany, France, U.K., Italy, Spain, Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Australia, Rest of Asia-Pacific), Latin America (Brazil, Mexico, Rest of Latin America), and the Middle East & Africa |
|
Key Companies |
Thermo Fisher Scientific Inc. (U.S.), Avioq, Inc. (U.S.), Creative Biolabs, Inc (U.S.), Maxim Biomedical, Inc. (U.S.), Bio-Techne Corporation (U.S.), Merck KGaA (Germany), PeploBio Ltd (U.K.), ICON plc (Ireland), NeoDx Biotech Labs Pvt. Ltd. (India), Eclevar Medtech (France), Savyon Diagnostics (Israel), Promega Corporation (U.S.), and Future Diagnostics Solutions B.V. (Netherlands). |
The global IVD assay development market size was valued at $4.60 billion in 2023.
The market is projected to grow from $4.93 billion in 2024 to $8.39 billion by 2031.
The IVD assay development market analysis indicates substantial growth, with projections indicating that the market will reach $8.39 billion by 2031 at a compound annual growth rate (CAGR) of 7.9% from 2024 to 2031.
The key companies operating in this market include Thermo Fisher Scientific Inc. (U.S.), Avioq, Inc. (U.S.), Creative Biolabs, Inc (U.S.), Maxim Biomedical, Inc. (U.S.), Bio-Techne Corporation (U.S.), Merck KGaA (Germany), PeploBio Ltd (U.K.), ICON plc (Ireland), NeoDx Biotech Labs Pvt. Ltd. (India), Eclevar Medtech (France), Savyon Diagnostics (Israel), Promega Corporation (U.S.), and Future Diagnostics Solutions B.V. (Netherlands).
Emerging economies and the use of advanced technologies in IVD assay development are expected to generate growth opportunities for market stakeholders.
By offering type, the IVD assay development services segment is forecasted to hold the largest market share during 2024-2031.
By application, the infectious diseases segment is expected to dominate the market during 2024-2031.
By technology, the immunoassay/immunochemistry segment is expected to dominate the market during 2024-2031.
By geography, North America is anticipated to hold the major market share during 2024-2031.
By region, North America holds the largest IVD assay development market share in 2024. However, the market in Asia-Pacific is expected to witness the highest growth rate, driven by continuous improvements in healthcare infrastructure and increasing government investment in the IVD sector. Moreover, accelerated economic growth in many Asia-Pacific countries has led governments to invest more in the healthcare sector to enhance accessibility to healthcare facilities and improve infrastructure in countries such as China, India, and South Korea.
Key factors driving the growth of this market include the rising prevalence of chronic diseases & the increasing geriatric population, the high burden of infectious diseases, the growing focus on the development of POC IVD assays, and rising healthcare expenditures.
1. Introduction
1.1. Market Definition & Scope
1.2. Market Ecosystem
1.3. Currency & Limitations
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Process
2.2. Data Collection & Validation
2.2.1. Secondary Research
2.2.2. Primary Research/Interviews with Key Opinion Leaders of the Industry
2.3. Market Sizing and Forecast
2.3.1. Market Size Estimation Approach
2.3.2. Growth Forecast Approach
2.3.3. Market Share Analysis
2.4. Assumptions for the Study
3. Executive Summary
4. Market Insights
4.1. Overview
4.2. Factors Affecting Market Growth
4.2.1. Drivers
4.2.1.1. Rising Prevalence of Chronic Diseases & the Increasing Geriatric Population
4.2.1.2. High Burden of Infectious Diseases
4.2.1.3. Growing Focus on the Development of POC IVD Assays
4.2.1.4. Rising Healthcare Expenditures
4.2.2. Restraints
4.2.2.1. Evolving Regulatory Landscape
4.2.3. Opportunities
4.2.3.1. Emerging Economies
4.2.3.2. Use of Advanced Technologies in IVD Assay Development
4.2.4. Challenges
4.2.4.1. Maintaining Product Quality & Protecting Proprietary Information
4.2.5. Macro Indicators
4.3. Adjacent Market Analysis
4.4. IVD Assay Development Market: Regulatory Landscape
4.4.1. North America
4.4.1.1. U.S.
4.4.1.2. Canada
4.4.2. Europe
4.4.3. Asia-Pacific
4.4.3.1. Japan
4.4.3.2. China
4.4.3.3. India
4.4.4. Latin America
4.4.5. Middle East & Africa
4.5. Porter’s Five Forces Analysis
4.5.1. Bargaining Power of Suppliers
4.5.2. Bargaining Power of Buyers
4.5.3. Threat of Substitutes
4.5.4. Threat of New Entrants
4.5.5. Degree of Competition
5. IVD Assay Development Market Assessment—by Offering
5.1. Overview
5.2. IVD Assay Development Services
5.3. IVD Assay Stability Testing
5.4. IVD Packaging Development Services
5.5. Regulatory Support
5.6. Other offerings
6. IVD Assay Development Market—by Technology
6.1. Overview
6.2. Immunoassay/Immunochemistry
6.3. Molecular Diagnostics
6.4. Clinical Chemistry/Biochemistry
6.5. Hematology
6.6. Microbiology
6.7. Urinalysis
7. IVD Assay Development Market Assessment—by Application
7.1. Overview
7.2. Infectious Diseases
7.3. Oncology
7.4. Diabetes
7.5. Cardiology
7.6. Other Applications
8. IVD Assay Development Market Assessment—by Geography
8.1. Overview
8.2. North America
8.2.1. U.S.
8.2.2. Canada
8.3. Europe
8.3.1. Germany
8.3.2. U.K.
8.3.3. France
8.3.4. Italy
8.3.5. Spain
8.3.6. Rest of Europe
8.4. Asia-Pacific
8.4.1. China
8.4.2. Japan
8.4.3. India
8.4.4. Australia
8.4.5. South Korea
8.4.6. Rest of Asia-Pacific
8.5. Latin America
8.5.1. Brazil
8.5.2. Mexico
8.5.3. Rest of Latin America
8.6. Middle East & Africa
9. Competition Analysis
9.1. Overview
9.2. Competitive Benchmarking
9.3. Competitive Dashboard
9.3.1. Industry Leaders
9.3.2. Market Differentiators
9.3.3. Vanguards
9.3.4. Emerging Companies
9.4. Market Ranking, by Key Players (2023)
10. Company Profiles (Business Overview, Financial Overview, Product Portfolio, Strategic Developments, And SWOT Analysis*)
10.1. Creative Biolabs, Inc.
10.2. Avioq, Inc.
10.4. Thermo Fisher Scientific Inc.
10.5. Neodx Biotech Labs Pvt. Ltd.
10.6. Merck KGaA
10.7. PeploBio Ltd
10.8. Icon Plc
10.9. Savyon Diagnostics
10.10. Maxim Biomedical, Inc.
10.11. Eclevar Medtech
10.12. Promega Corporation
10.13. Future Diagnostics Solutions B.V.
(Note: SWOT Analysis of the Top 5 Companies Will Be Provided)
11. Appendix
11.1. Available Customization
11.2. Related Reports
List of Tables
Table 1 Healthcare Expenditure per Capita, by Country, 2021/2022 (USD)
Table 2 Global Human Immunodeficiency Virus (HIV) Statistics, 2023
Table 3 Percentage of the Population Aged 65 Years or over, by Region, 2022 VS. 2030 VS. 2050
Table 4 Global IVD Assay Development Market, by Offering, 2022-2031 (USD Million)
Table 5 Global IVD Assay Development Services Market, by Country/Region, 2022-2031 (USD Million)
Table 6 Global IVD Assay Stability Testing Market, by Country/Region, 2022-2031 (USD Million)
Table 7 Global IVD Packaging Development Services Market, by Country/Region, 2022-2031 (USD Million)
Table 8 Global IVD Assay Development Regulatory Support Market, by Country/Region, 2022-2031 (USD Million)
Table 9 Global Other IVD Assay Development Offerings Market, by Country/Region, 2022-2031 (USD Million)
Table 10 Global IVD Assay Development Market, by Technology, 2022-2031 (USD Million)
Table 11 Global IVD Assay Development Market for Immunoassay/ Immunochemistry, by Country/Region, 2022-2031 (USD Million)
Table 12 Global IVD Assay Development Market for Molecular Diagnostics, by Country/Region, 2022-2031 (USD Million)
Table 13 Global IVD Assay Development Market for Clinical Chemistry/Biochemistry, by Country/Region, 2022-2031 (USD Million)
Table 14 Global IVD Assay Development Market for Hematology, by Country/Region, 2022-2031 (USD Million)
Table 15 Global IVD Assay Development Market for Microbiology, by Country/Region, 2022-2031 (USD Million)
Table 16 Global IVD Assay Development Market for Urinalysis, by Country/Region, 2022-2031 (USD Million)
Table 17 Global IVD Assay Development Market, by Application, 2022-2031 (USD Million)
Table 18 Global IVD Assay Development Market for Infectious Diseases, by Country/Region, 2022-2031 (USD Million)
Table 19 Global IVD Assay Development Market for Oncology, by Country/Region, 2022-2031 (USD Million)
Table 20 Number of People with Diabetes Aged 20–79 Years, BY Region, 2021 VS. 2030 VS. 2045 (In Million)
Table 21 Global IVD Assay Development Market for Diabetes, by Country/Region, 2022-2031 (USD Million)
Table 22 Global IVD Assay Development Market for Cardiology, by Country/Region, 2022-2031 (USD Million)
Table 23 Global IVD Assay Development Market for Other Applications, by Country/Region, 2022-2031 (USD Million)
Table 24 Global IVD Assay Development Market, by Country/Region, 2022–2031 (USD Million)
Table 25 North America: IVD Assay Development Market, by Country, 2022-2031 (USD Million)
Table 26 North America: IVD Assay Development Market, BY offering, 2022–2031 (USD Million)
Table 27 North America: IVD Assay Development Market, BY Technology, 2022–2031 (USD Million)
Table 28 North America: IVD Assay Development Market, BY Application, 2022–2031 (USD Million)
Table 29 U.S.: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 30 U.S.: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 31 U.S.: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 32 Canada: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 33 Canada: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 34 Canada: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 35 Europe: IVD Assay Development Market, by Country/Region, 2022-2031 (USD Million)
Table 36 Europe: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 37 Europe: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 38 Europe: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 39 Germany: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 40 Germany: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 41 Germany: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 42 Number of Incidents of Influenza and Other Respiratory Diseases in England, by Institution and Virus Type, Winter 2022 To 2023
Table 43 U.K.: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 44 U.K.: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 45 U.K.: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 46 France: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 47 France: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 48 France: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 49 Italy: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 50 Italy: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 51 Italy: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 52 Spain: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 53 Spain: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 54 Spain: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 55 Roe: Estimated Number of New Cancer Cases, by Country (2022 Vs. 2030)
Table 56 Rest of Europe: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 57 Rest of Europe: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 58 Rest of Europe: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 59 Asia-Pacific: IVD Assay Development Market, by Country/Region, 2022-2031 (USD Million)
Table 60 Asia-Pacific: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 61 Asia-Pacific: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 62 Asia-Pacific: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 63 China: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 64 China: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 65 China: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 66 Japan: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 67 Japan: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 68 Japan: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 69 India: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 70 India: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 71 India: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 72 Australia: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 73 Australia: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 74 Australia: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 75 South Korea: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 76 South Korea: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 77 South Korea: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 78 Rest of Asia-Pacific: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 79 Rest of Asia-Pacific: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 80 Rest of Asia-Pacific: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 81 Latin America: IVD Assay Development Market, by Country/Region, 2022-2031 (USD Million)
Table 82 Latin America: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 83 Latin America: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 84 Latin America: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 85 Brazil: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 86 Brazil: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 87 Brazil: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 88 Mexico: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 89 Mexico: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 90 Mexico: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 91 Rest of Latin America: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 92 Rest of Latin America: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 93 Rest of Latin America: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 94 Middle East & Africa: IVD Assay Development Market, by Offering, 2022–2031 (USD Million)
Table 95 Middle East & Africa: IVD Assay Development Market, by Technology, 2022–2031 (USD Million)
Table 96 Middle East & Africa: IVD Assay Development Market, by Application, 2022–2031 (USD Million)
Table 97 Global IVD Assay Development Market Rankings, by Key Players, 2023
List of Figures
Figure 1 Research Process
Figure 2 Secondary Sources Referenced for This Study
Figure 3 Primary Research Techniques
Figure 4 Key Executives Interviewed
Figure 5 Breakdown of Primary Interviews (Supply-Side & Demand-Side)
Figure 6 Market Sizing and Growth Forecast Approach
Figure 7 Global IVD Assay Development Market, by offering, 2024 Vs.2031 (USD Million)
Figure 8 Global IVD Assay Development Market, by Technology, 2024 Vs. 2031 (USD Million)
Figure 9 Global IVD Assay Development Market, by Application, 2024 Vs. 2031 (USD MILLION)
Figure 10 Global IVD Assay Development Market, by Geography, 2024 VS 2031 (USD Million)
Figure 11 Impact Analysis of Market Dynamics
Figure 12 GLOBAL Biopharmaceutical R&D Expenditure, 2016–2026 (USD Billion)
Figure 13 Estimated Number of New Cancer Cases, by Region, 2022 VS. 2030
Figure 14 Number of People with Diabetes Aged 20–79 Years, by Region, 2021 VS. 2030 VS. 2045 (in Million)
Figure 15 IVD Market Share, BY Technology, 2024
Figure 16 IVD Reagents Market, by Type, 2024 Vs. 2031 (USD Million)
Figure 17 IVD Contract Manufacturing Services Market, by Type, 2024 Vs. 2031 (USD Million)
Figure 18 Porter’s Five Forces Analysis
Figure 19 Global IVD Assay Development Market, by offering, 2024 VS. 2031 (USD Million)
Figure 20 IVD Assay Development Phases
Figure 21 Global IVD Assay Development Market, by Technology, 2024 VS. 2031 (USD Million)
Figure 22 Global IVD Assay Development Market, by Application, 2024 VS. 2031 (USD Million)
Figure 23 Estimated Number of New Cancer Cases, by Region, 2022–2030
Figure 24 Global IVD Assay Development Market, by Country/Region, 2024 Vs. 2031 (USD Million)
Figure 25 North America: IVD Assay Development Market Snapshot
Figure 26 Europe: IVD Assay Development Market Snapshot
Figure 27 France: Percentage of the Population Aged 65 Years and Above (2016–2020)
Figure 28 Number of HIV Tests Performed in Catalonia (Spain), 2014–2021
Figure 29 Asia-Pacific: IVD Assay Development Market Snapshot
Figure 30 China: Independent Medical Laboratories Market Size (USD Million)
Figure 31 Japan: Percentage of Aging Population (65 Years and Above), 2016-2050
Figure 32 Latin America: IVD Assay Development Market Snapshot
Figure 33 IVD Assay Development Market: Competitive Benchmarking (Based on Offering)
Figure 34 IVD Assay Development Market: Competitive Benchmarking (Based on Geography)
Figure 35 Competitive Dashboard: IVD Assay Development Market
Figure 36 Bio-Techne Corporation: Financial Snapshot (2023)
Figure 37 Thermo Fisher Scientific Inc.: Financial Snapshot (2023)
Figure 38 Merck KGaA: Financial Snapshot (2023)
Figure 39 ICON Plc: Financial Snapshot (2023)
Global IVD Assay Development Market Will Grow at A CAGR of 7.9% during the Forecast Period to Reach $8.39 Billion by 2031, Says “Meticulous Research®”
IVD assay development is the process of creating, refining, and verifying diagnostic assays that identify and quantify pathogens, biomarkers, and other substances present in biological samples. In healthcare settings, these tests are utilized for the diagnosis, monitoring, and management of various diseases and conditions.
The growth of this market is driven by the rising prevalence of chronic diseases & the increasing geriatric population, the high burden of infectious diseases, the growing focus on the development of POC IVD assays, and rising healthcare expenditures. Additionally, emerging economies and the use of advanced technologies in IVD assay development are expected to offer significant market growth opportunities.
Here are the top 13 companies operating in the global IVD assay development market:
Creative Biolabs, Inc. (U.S.)
Founded in 2005 and headquartered in New York, U.S., Creative Biolabs, Inc. provides distinctive pharmaceutical and biotechnology services that address the complete range of biotechnology demands, including early discovery and drug development. The company focuses on the development of novel antibodies or their equivalents that possess suitable specificity and affinity for applications in research, therapeutics, and diagnostics.
The company provides a comprehensive range of services, including Phage Display Library Construction, Phage Display Library Screening, Magic Anti-Membrane Protein Antibody Discovery, Hybridoma Services, Anti-idiotype Antibody Discovery, Single Domain Antibody (sdAb) Discovery, Native Antibody Discovery, De Novo Antibody Sequencing, Antibody Humanization, Antibody Affinity Maturation, Post-translational Modification (PTM) Specific Antibody Discovery, as well as IVD Antibody Development Platforms, IVD Kit Development, IVD Materials and Reagents Development Services, Custom Antibody Array Development Services, Custom IVD Antibody Production Services, Custom Formulation Development Services, Custom Assay Development Services, and Biomarker Analysis Services. Additionally, the company offers platforms such as the Membrane Protein Platform, Phage Display Platform, Hyperdoma Platform, and B-Cell Sorting Platform to government and academic research laboratories, as well as pharmaceutical and biotechnology companies worldwide.
The company engages in the IVD assay development market by providing various services, including radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), lateral-flow immunochromatographic assay (LFIA), western blot (WB), latex particle-enhanced turbidimetric immunoassay (LETIA), immunohistochemistry (IHC), and flow cytometry (FC). Creative Biolabs maintains a global presence with offices and distributors located in the U.K., the U.S., China, Germany, Australia, Belgium, Chile, France, Japan, South Korea, the Netherlands, Switzerland, Spain, South Africa, Singapore, and Italy. The company provides a comprehensive EU, GMP, USP, and FDA-compliant testing laboratory, offering analytical and bioassay services.
Avioq, Inc. (U.S.)
Founded in 2007 and headquartered in North Carolina, U.S., Avioq, Inc. is engaged in the development, production, and distribution of IVD devices and products. The company offers services for IVD assay development, IVD contract manufacturing, cell culture development, companion diagnostics, and direct-to-consumer testing. The company operates in the IVD assay development market by offering a range of IVD assay development services. It has established a presence through manufacturing sites, distributors, and offices in the U.S. and Europe.
Bio-Techne Corporation (U.S.)
Founded in 1976 and headquartered in Minnesota, U.S., Bio-Techne Corporation develops, manufactures, and sells life science reagents, instruments, and services within the research and clinical diagnostic markets. The company provides services, including custom controls and calibrators, assay development, IVD manufacturing, technology transfer, packaging development, regulatory support, technical support, and stability programs. BioTechne operates in two segments: Protein Sciences and Diagnostics & Genomics, with its involvement in the IVD assay development market occurring through the Diagnostics & Genomics segment.
The Genomics & Diagnostics segment develops and manufactures diagnostic products, including calibrators, controls, and diagnostic assays for regulated diagnostics, advanced tissue-based in-situ hybridization, exosome-based molecular diagnostic assays for spatial genomic and tissue biopsy analysis, and genetic and oncology kits for clinical and research applications.
The company maintains a direct presence through subsidiaries, manufacturing sites, sales offices, and distributors across the U.K., U.S., Canada, China, India, and France. Notable subsidiaries involved in the IVD assay development market include Bionostics, Inc. (U.S.), Bio-Techne China Co. Ltd (China), Bio-Techne SAS (France), Bio-Techne GmbH (Germany), Bio-Techne France (France), Bio-Techne AG (Switzerland), Bio-Techne srl (Italy), and Bio-Techne Ireland Limited (Ireland)
Thermo Fisher Scientific Inc. (U.S.)
Founded in 1956 and headquartered in Massachusetts, U.S., Thermo Fisher Scientific Inc. is a biotechnology company focused on life sciences research, enhancing patient diagnostics, and boosting laboratory productivity. The company operates through four reportable segments: Life Sciences Solutions, Analytical Instruments, Specialty Diagnostics, and Laboratory Products & Biopharma Services.
The Specialty Diagnostics segment consists of five business categories: Clinical Diagnostics, ImmunoDiagnostics, Microbiology, Transplant Diagnostics, and Healthcare Market Channel. The company participates in the IVD assay development market through its Clinical Diagnostics business, offering detection assays, liquid and lyophilized ready-to-use immunodiagnostic reagent kits, calibrators, controls, proteins, and instruments.
Key brands within Thermo Fisher include Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services, Patheon, and PPD. The company maintains a direct presence through regional offices and manufacturing facilities located in the U.S., Mexico, Brazil, Germany, Belgium, Austria, the U.K., Italy, the Netherlands, South Africa, the UAE, India, China, Japan, Australia, and the Republic of Korea. Notable subsidiaries active in the IVD assay development market comprise Thermo Fisher Scientific Operating Company LLC (Delaware), Fisher Clinical Services Japan K.K. (Japan), Fisher Clinical Services (Korea) Co., Ltd (Korea), and Thermo Fisher Scientific SL (Spain).
NeoDx Biotech Labs Pvt. Ltd. (India)
Incorporated in 2020 and headquartered in Karnataka, India., NeoDx Biotech Labs Pvt. Ltd. is engaged in developing, designing, and manufacturing IVD kits and provides services for diagnosing infectious diseases. The company’s in vitro diagnostic products encompass kits, reagents, and devices for both infectious and noninfectious diseases. Additionally, it offers various self-test kits such as NeoCheck to test for infectious disorders and Hana Health for women seeking fertility solutions.
The company offers various services, such as contract research, assay development, quality and regulatory, OEM manufacturing contract research, and manufacturing services. It operates in the IVD assay development market by offering assay development services.
Merck KGaA (Germany)
Founded in 1668 and headquartered in Darmstadt, Germany, Merck KGaA is a science and technology company that aims to improve people's health and well-being and advance their lives through science and technology. The company operates in three reportable segments: Healthcare, Life Science, and Electronics. The Life Science segment has three categories: Process Solutions, Life Science Services, and Science & Lab Solutions. The company operates in the IVD assay development market through the Science & Lab Solutions category by offering lateral flow assay development services.
The company's Life Science business segment has more than 55 manufacturing sites worldwide and over 100 distribution centers. The company has corporate offices, manufacturing units, and distributors in Argentina, Germany, Malaysia, Singapore, France, Romania, Australia, New Zealand, Sweden, Switzerland, the U.K., Colombia, Japan, Brazil, Belgium, the Netherlands, Croatia, the U.S., and Iraq.
Some of its subsidiaries operating in the IVD assay development market are Merck Life Science Germany GmbH (Germany), Merck Millipore Ltd. (Ireland), Merck Life Science S.L.U. (Spain), Sigma-Aldrich International GmbH (Switzerland), Sigma-Aldrich Biochemie GmbH (Germany), Merck Life Science S.r.l. (Italy), and Merck Life Science AS (Norway).
PeploBio Ltd (U.K)
Incorporated in 2021 and headquartered in Guildford, U.K., PeploBio Ltd is a clinical diagnostic laboratory and full-service contract research organization (CRO) that offers a comprehensive range of products and services to facilitate the development of medical therapeutics and devices. Their service offerings include clinical trials, product development, report preparation, immune monitoring, as well as genomic, proteomic, and diagnostic services.
The company specializes in Research Use Only (RUO), Laboratory-developed Tests (LDT), IVD, and CDx assays, and emerging technologies such as PCR, cell-based assays, flow cytometry, sanger sequencing, ELISA, and lateral flow. It offers diagnostic testing solutions in the fields of infectious disease, oncology, serology, and more.
ICON plc (Ireland)
Founded in 1990 and headquartered in Dublin, Ireland. ICON plc offers outsourced development services to biotechnology, pharmaceutical, medical devices, and government/public health organizations. The company operates within a single reportable segment focused on delivering these outsourced development services.
The company maintains a direct presence in over 40 countries, operating 39 offices in Europe, including 10 in the U.K., five in the Netherlands, four in both Germany and Spain, two each in France, Ireland, Hungary, Poland, Sweden, Russia, Turkey, and Ukraine, and one each in Belgium, Belarus, Bulgaria, Georgia, the Czech Republic, Israel, Latvia, Romania, Italy, and Slovakia.
In North America, the company has 33 offices, with 29 located in the U.S., two in Canada, and two in Mexico. In Asia, there are 16 offices, including five in China (with one in Hong Kong), three in India, two each in Japan and Singapore, and one each in South Korea, Thailand, Taiwan, and the Philippines. Additionally, the company operates two offices in Australia and five offices in South America, with one office each in Argentina, Chile, Brazil, Colombia, and Peru, as well as one office in South Africa.
Several of its subsidiaries operating in the IVD assay development market include ICON Clinical Research Austria GmbH (Austria), ICON Laboratory Services, Inc. (U.S.), ICON Chile Limitada (Chile), ICON CRO Malaysia Sdn. Bhd. (Malaysia), ICON Clinical Research Korea Limited (South Korea), ICON Life Sciences Canada Inc. (Canada), and ICON Clinical Research Services Philippines, Inc. (Philippines).
Savyon Diagnostics (Israel)
Founded in 1984 and headquartered in Ashdod, Israel, Savyon Diagnostics specializes in the development and manufacturing of diagnostic tests for point-of-care, clinical laboratories, and home use. Its Analytical Unit provides a comprehensive suite of services to the pharmaceutical, biotech, IVD, and life sciences sectors, including assay development, contract manufacturing, logistics, and quality assurance & regulatory consulting. The company also maintains a global network of over 80 distributors.
Maxim Biomedical, Inc. (U.S.)
Founded in 2005 and headquartered in Maryland, U.S., Maxim Biomedical, Inc. is a diagnostic healthcare company that develops and manufactures diagnostic solutions. The company provides a comprehensive range of testing solutions for HIV surveillance, screening, confirmation, and diagnosis. Additionally, it specializes in custom IVD assay development for both government and private sector clients.
























Published Date: Apr-2025
Published Date: Feb-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Aug-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates