Resources
About Us
Hemostats Market Size, Share, Forecast, & Trends Analysis by Type (Gelatin, Collagen, Thrombin, Fibrin, Synthetic Sealant, Gauze) Form (Sheet, Powder, Gel) Application (Orthopedic, General, Cardiovascular, Dental, Trauma) End User - Global Forecast to 2032
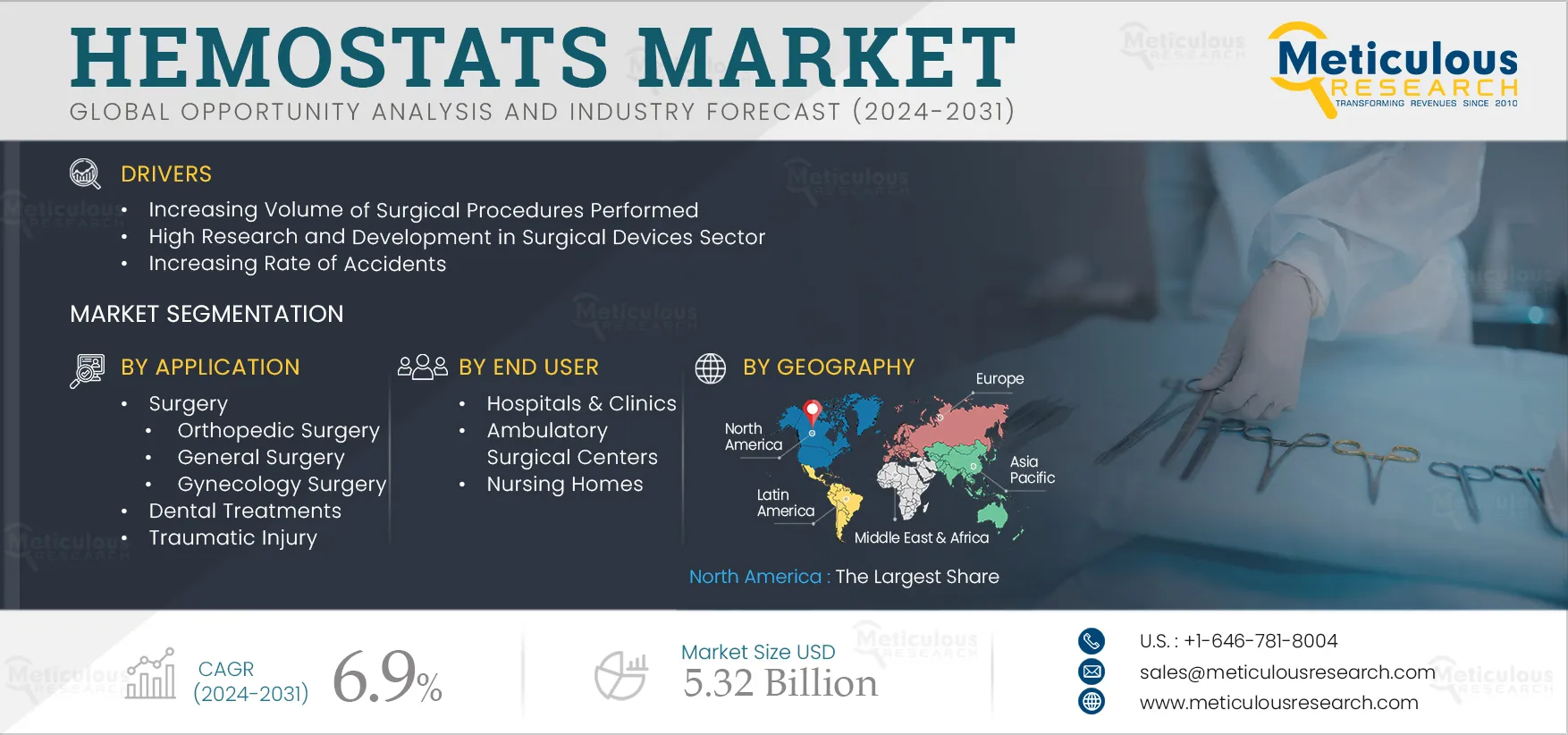
Report ID: MRHC - 1041150 Pages: 280 Jan-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe growth of this market is driven by the increasing prevalence of chronic diseases such as cancer, cardiovascular, orthopedic, and neurological diseases and subsequent increase in the volume of surgical procedures; increasing rate of accidents; rise in the number of cesarean deliveries, fatal injuries, and organ transplantation; increased R&D activities; rising healthcare spending; and growing preference for minimally invasive surgical procedures. Additionally, the increasing use of advanced hemostats, advancements in dental hemostatic agents, and increasing demand for preloaded applicators and ready-to-use hemostats are expected to offer significant market growth opportunities.
However, allergic reactions caused by the material used in making hemostats restrain the growth of this market. Additionally, the high manufacturing price for hemostats poses a significant challenge to the market's growth. Furthermore, biopolymer-based hemostats and dual-action hemostatic technology are trends prevailing in the hemostats market.
The rate of accidents has increased over the years. As per the U.S. Department of Health & Human Services, January 2024 report, road traffic crashes are among the leading causes of death in the U.S. Similarly, approximately 20,653 people were killed in road accidents in the EU, an increase of 3.7% compared to 2021 (Source: European Commission, Directorate-General for Communication). The increasing number of accidents has increased the number of hospital visits by patients for minor and major surgeries and wound care, driving the demand for hemostatic products in hospitals & clinics.
The rate of minimally invasive surgeries (MIS) has been increasing in recent years. These surgeries necessitate tiny incisions, which creates demand for advanced hemostatic agents or tamponing agents to control blood loss.
With developments in minimally invasive surgery (MIS) approaches and technologies, MIS is becoming more popular in a variety of surgical disciplines, particularly cardiac surgery for coronary artery bypass surgery (CABG), valve surgery, and orthopedic surgery for hip and knee replacements. Furthermore, the adoption of minimally invasive surgeries is growing due to the benefits offered over traditional surgeries, such as easy and faster blood flow control, leading to shorter healing time and hospital stay, fewer complications, and less pain. Hemostats act as a powerful tool for surgeons to achieve hemostasis in these complicated surgeries. According to the American Society of Plastic Surgeons, in 2022, 682,932 non-invasive fat reduction surgeries and 408,970 non-invasive skin tightening surgeries were conducted in the U.S. Minimally invasive techniques frequently require operating complex cases as doctors limit the size and number of cuts or incisions. Hemostats created expressly for these procedures provide precision bleeding control, enabling surgeons to accomplish correct hemostasis.
Click here to: Get Free Sample Pages of this Report
Biopolymer-based hemostats are becoming popular in surgical hemostasis. These materials may assist with bleeding and come in a variety of forms, including particles, powder, sponges, sheets, foams, films, and hydrogels. Biopolymers have proven their applications as hemostatic materials due to their capabilities as biodegradable, biocompatible, and bioactive.
Biopolymer-based hemostats are meant to be biocompatible, which means the body easily absorbs them and does not trigger adverse reactions. The materials implemented in hemostats are selected with care to reduce the chance of immunological reactions and inflammation. Biopolymer-based hemostats provide a possible alternative to conventional hemostatic approaches. Their biocompatibility, absorbability, and hemostatic effectiveness make them particularly suitable choices for surgical treatments. As biopolymer technology research and development progresses, it is expected to bring more advances and higher adoption of biopolymer-based hemostats in clinical practice.
Dual-action hemostatic technology effectively and promptly controls gingival bleeding. This technology approach ensures a comprehensive solution for managing bleeding during dental treatments. Dual-action hemostatic technology characteristics include effectivity and reliability, pose no biological risk in-vivo, are free from side effects, and stop bleeding quickly. Dual-action hemostatic technology achieves hemostasis in deep and complex wounds with secluded hemorrhagic sites. Additionally, the sealing capabilities provide suture support in vascular surgery, which is highly demanded by surgeons. Additionally, the rising preference for these hemostats is due to their dual mechanism of action, which involves the interaction of two different components which include collagen mediation of intrinsic hemostatic action liable to form fibrin clots and speedy adherence to applied tissue due to the electrophilic cross-linking with a protein-reactive polyethylene glycol monomer. Such advantages of dual-action hemostatic technology are expected to drive the hemostats market.
The use of hemostat products in dental procedures is a growing area of interest due to the advantages offered by various hemostatic agents, including gelatin-based hemostatic agents, owing to their biocompatibility, biodegradability, and low cost of raw materials. Many dental procedures, including exodontia, endosseous implantation, tissue biopsies, and periodontal surgery, have benefited due to the use of hemostats to control blood flow and its ability to reach inaccessible areas. Additionally, hemostats have been flexible to adapt to the wound and be removed easily without disintegrating into the bloodstream, owing to their increased demand in dental procedures. Furthermore, hemostats have been highly preferred by healthcare professionals to reduce increased healthcare expenditures for the patient and avoid unwanted postsurgical repair/infections associated with uncontrolled surgical bleeding. These factors have contributed to the growth of hemostats in dental procedures.
Based on type, the hemostats market is segmented into absorbable hemostats and non-absorbable hemostats. In 2025, the absorbable hemostats segment is expected to account for the largest share of 58.6% of the hemostats market. The segment’s large share is attributed to the advantages offered by absorbable hemostats, such as controlling bleeding from fistula-puncture sites, achieving hemostasis during vascular surgery, controlling capillary bleeding, availability of versatile forms of hemostats, and rapid embolization.
However, the non-absorbable hemostats segment is projected to witness the highest growth rate of 7.6% during the forecast period of 2022–2032. This growth is driven by the growing use of fibrin sealants as hemostats by surgeons during surgery to physically provide an effective barrier for controlling blood flow. These sealants offer high biocompatibility, less time needed for clot formation, and the use of non-absorbable hemostats in high blood flow cases.
Based on application, the hemostats market is segmented into surgery, dental treatments, and traumatic injury. In 2025, the surgery segment is expected to account for the largest share of the hemostats market. The segment’s large share is attributed to the recurrent use of various hemostatic agents in the increasing number of orthopedic, general, gynecological, and cardiovascular surgeries, the rising number of accidents and traumatic injuries leading to surgical treatments, and the use of advanced hemostats and advance sealants by healthcare professionals in minimally invasive surgeries for controlling the blood flow.
Based on form, the hemostats market is segmented into sponges and dressings, sheets and pads, powder, tools, and matrix and gel. In 2025, the matrix and gel segment is expected to account for the largest share of 41.3% of the hemostats market. The segment’s large share is attributed to advantages offered by matrix and gel hemostatic agents, such as the formation of a physical matrix, promoting activation of the clotting pathway; their wide applications in patients with intact coagulation cascade; availability of bioactive and biocompatible gels; and cost efficiency.
This segment is also projected to witness the highest growth rate of 7.2% during the forecast period of 2022–2032.
Based on hospitals & clinics, the hemostats market is segmented into hospitals & clinics, ambulatory surgical centers, and nursing homes. In 2025, the hospitals & clinics segment is expected to account for the largest share of the hemostats market. The significant market share of this segment is attributed to the rising number of patient visits for surgical procedures and traumatic injury treatment in hospitals, the increasing number of surgeries, the willingness to spend on advance hemostats by hospitals, and favorable reimbursement policies.
In 2025, North America is expected to account for the largest share of 32.9% of the hemostats market, followed by Europe and Asia-Pacific. In North America, the U.S. accounted for the largest share in 2025. The significant market share of the U.S. can be attributed to several key factors including the presence of key market players in the U.S., a well-established and technologically advanced healthcare infrastructure, and favorable government policies in the country.
Moreover, the market in Asia-Pacific is slated to register the highest growth rate (CAGR 7.8%) due to significantly growing markets such as India and China. The growth of this market is attributed to an increase in the number of acute injuries and accidents, an increase in surgical procedures in the countries, rising medical tourism, rising healthcare expenditures, and the focus of major players in these countries due to increasing market potential.
The report offers a competitive analysis based on an extensive assessment of the leading players’ product portfolios, geographic presence, financial growth, and key growth strategies adopted in the last 3–4 years. Some of the key players operating in the hemostats market are Baxter International Inc. (U.S.), Pfizer Inc. (U.S.), B. Braun Melsungen AG (Germany), C. R. Bard, Inc.(U.S.), Integra LifeSciences (U.S.), Medtronic plc (Ireland), CSL Behring (U.K.), Gelita Medical GmbH (Germany), Grifols, S.A. (Spain), Abbott Laboratories (U.S.), CryoLife (U.S.), Ethicon, Inc. (U.S.) (Subsidiary of Johnson & Johnson.), Medtronic plc (Ireland), Becton, Dickinson and Company (BD) (U.S.), and Advanced Medical Solutions Group plc (U.K.). The Hemostats market is consolidated in nature, and the top 5-6 players accounted for a combined share of 40-50% in 2024.
|
Particulars |
Details |
|
Number of Pages |
~ 280 |
|
Format |
|
|
Forecast Period |
2025–2032 |
|
Base Year |
2024 |
|
CAGR (Value) |
6.9% |
|
Market Size (Value) |
USD 5.32 Billion by 2032 |
|
Segments Covered |
By Type
By Application
By Form
By End User
|
|
Countries Covered |
North America (U.S., Canada), Europe (France, Germany, U.K., Italy, Spain, Switzerland, Netherlands, and Rest of Europe), Asia-Pacific (China, India, Japan, Australia, South Korea, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East & Africa |
|
Key Companies |
Baxter International Inc. (U.S.), Pfizer Inc. (U.S.), B. Braun Melsungen AG (Germany), C. R. Bard, Inc. (U.S.), Integra LifeSciences (U.S.), Medtronic plc (Ireland), CSL Behring (U.K.), Gelita Medical GmbH (Germany), Grifols, S.A. (Spain), Abbott Laboratories (U.S.), CryoLife (U.S.), Ethicon, Inc. (U.S.) (Subsidiary of Johnson & Johnson.), Medtronic plc (Ireland), Becton, Dickinson and Company (BD) (U.S.), and Advanced Medical Solutions Group plc (U.K.). |
This study offers a detailed assessment of the hemostats market, including the market sizes & forecasts for market segments such as type, application, form, and end user. It also provides an in-depth analysis of various segments & subsegments of the hemostats market at the regional and country levels.
The hemostats market is projected to reach $5.32 billion by 2032, at a CAGR of 6.9% during the forecast period.
In 2025, the absorbable hemostats segment is expected to account for the largest market share. This segment's significant market share is attributed to its low cost, no risk of blood-borne diseases, super absorbent capacity, and use in dental procedures.
In 2025, the surgery segment is expected to provide high growth, which can be attributed to factors such as the increasing number of surgeries in hospitals and ambulatory surgery centers, the increasing number of accidents, and the rise in cosmetic/plastic surgical procedures.
The growth of this market is driven by the increasing volume of surgical procedures performed, high research and development in the surgical devices sector, increasing rate of accidents, increase in cesarean deliveries, fatal injuries, and organ transplantation, increased R&D activities by government organizations and the private sector, rising government healthcare spending, and growing adoption of minimally invasive surgical procedures.
The key players profiled in the Hemostats market study are Baxter International Inc. (U.S.), Pfizer Inc. (U.S.), B. Braun Melsungen AG (Germany), C. R. Bard, Inc.(U.S.), Integra LifeSciences (U.S.), Medtronic plc (Ireland), CSL Behring (U.K.), Gelita Medical GmbH (Germany), Grifols, S.A. (Spain), Abbott Laboratories (U.S.), CryoLife (U.S.), Ethicon, Inc. (U.S.) (Subsidiary of Johnson & Johnson.), Medtronic plc (Ireland), Becton, Dickinson and Company (BD) (U.S.), and Advanced Medical Solutions Group plc (U.K.).
Countries such as India and China are expected to offer significant growth opportunities for the vendors in this market due to factors such as the increasing adoption of hemostat solutions for surgical procedures, rising healthcare expenditures along with medical tourism in these countries, and the increasing number of accidents and chronic injuries.
























Published Date: Apr-2023
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Feb-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates