Resources
About Us
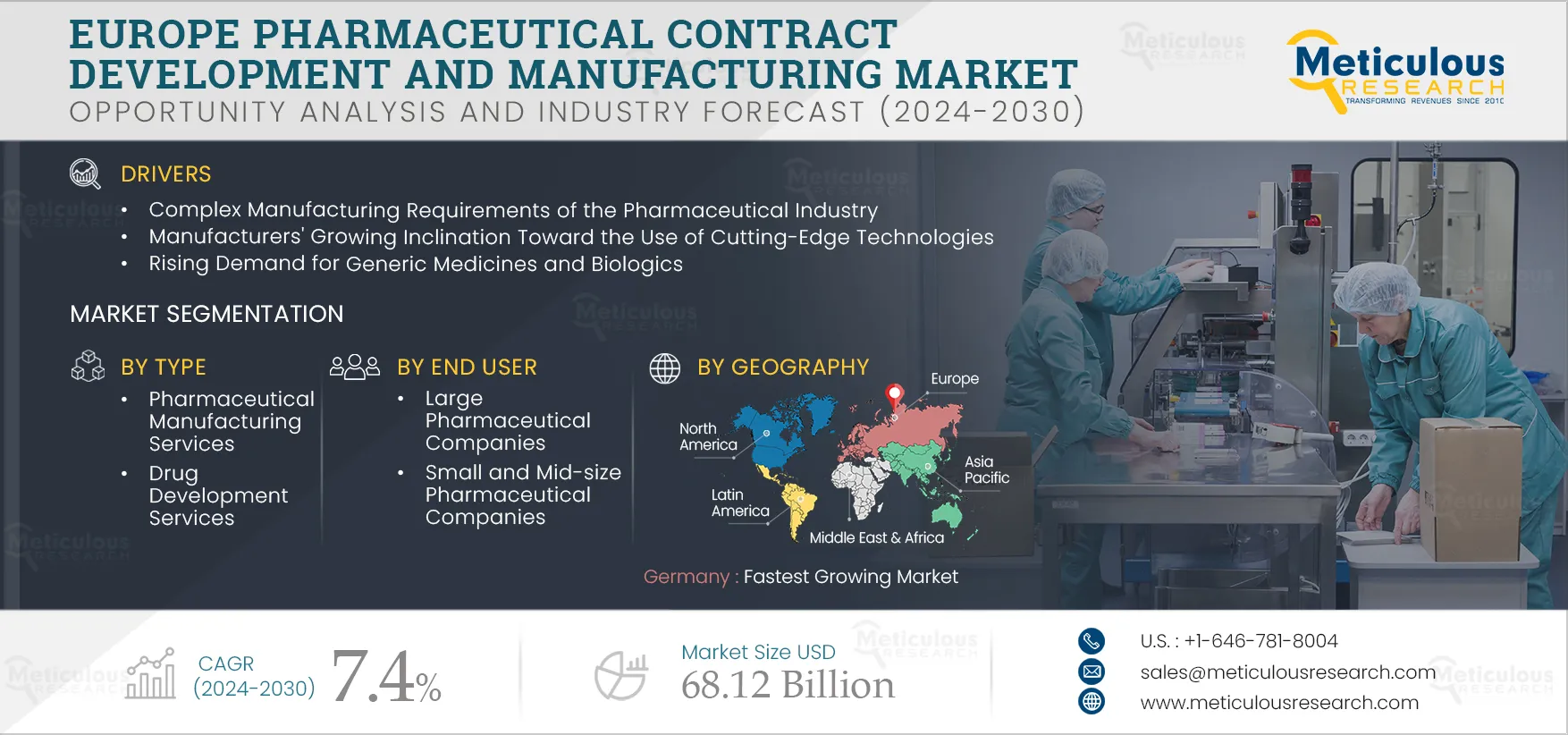
Europe Pharmaceutical Contract Development and Manufacturing Market by Service {Manufacturing [API, FDF (Parenteral, Tablet, Capsule, Oral Liquid)], Drug Development, Biologics Manufacturing, Packaging}, End User [Large Pharma, Generic] - Forecast to 2032
Report ID: MRHC - 104909 Pages: 150 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Europe Pharmaceutical Contract Development and Manufacturing Market is expected to reach $68.12 billion by 2032 at a CAGR of 7.4% from 2025 to 2032. Pharmaceutical contract development and manufacturing organizations offer various services based on a contract to small or large pharmaceutical companies. These services include active pharmaceutical ingredient (API) manufacturing, finished dosage form (FDF) manufacturing, drug development & drug discovery services, and biologics manufacturing services. The COVID-19 pandemic positively impacts the pharmaceutical contract development and manufacturing market due to the increased demand for pharmaceutical products such as tablets, capsules, drugs, and vaccines.
The growth of the Europe pharmaceutical contract development and manufacturing market is attributed to the complex manufacturing requirements of the pharmaceutical industry, manufacturers' growing inclination toward the use of cutting-edge technologies, consistent growth in the pharmaceutical industry due to growing government support, and the rising demand for generic medicines & biologics. In addition, the growing demand for cell and gene therapies and personalized medicines and growth in high potency active pharmaceutical ingredients (HPAPI) and antibody-drug conjugates (ADC) markets is expected to offer significant opportunities for the growth of the pharmaceutical contract development and manufacturing market. However, the lack of skilled professionals is challenging the growth of this market.
The pharmaceutical industry is largely driven by scientific discovery, development, and toxicological and clinical experience. Pharmaceutical manufacturing operations may be categorized as primary production of bulk drug substances (APIs) and manufacturing of dosage form products (formulations).
In addition to the complexity of the pharmaceutical manufacturing process, many countries globally have specific legal protections for proprietary drugs and manufacturing processes, known as intellectual property rights. Some companies specialize in manufacturing and marketing generic drugs when legal protections are limited or nonexistent. The pharmaceutical industry requires large capital investments due to the high expenses associated with R&D, regulatory approvals, manufacturing, quality assurance & control, marketing, and sales.
Many countries have extensive government regulations affecting the development and approval of drugs for commercial sale. These countries have strict requirements for good manufacturing practices to ensure the integrity of drug manufacturing operations and the quality, safety, and efficacy of pharmaceutical products. These challenges have led pharmaceutical companies to partner with contract manufacturing and development organizations to develop and manufacture pharmaceuticals and biologics, driving the growth of the pharmaceutical contract development and manufacturing market.
Click here to: Get a Free Sample Copy of this Report
Increasing Investment in Pharmaceutical R&D is Driving the Market
Healthcare R&D activities have significantly increased in the last two to three decades with rising investments from various government organizations and pharmaceutical companies. This funding is mainly driven by the rising prevalence of chronic diseases due to the growing geriatric population, complexities in clinical trials, and higher failure of drugs in early-phase studies. Governments in European countries are increasing their funding for boosting pharmaceutical and biopharmaceutical research. In addition, several initiatives have been taken by the government of respective countries to boost pharmaceutical R&D. According to the European Federation of Pharmaceutical Industries and Associations (EFPIA), pharmaceutical R&D increased to USD 49,102 million in 2021 from USD 45,295.2 million in 2020.
Furthermore, pharmaceutical companies have also increased their spending on R&D. The extent of pharmaceutical R&D spending is an important metric to show a company's commitment to finding new drugs. Therefore, increasing government investments in pharmaceutical R&D is expected to expand the research pipeline, enhancing the number of products converted to usable forms with the help of pharmaceutical contract manufacturers and driving market growth.
In Europe, Germany has the highest investment in pharmaceutical research. According to the European Federation of Pharmaceutical Industries and Associations (EFPIA), in 2020, Germany invested USD 6,850 million (EUR 7,813 million) in R&D. The increasing investment in R&D by pharmaceutical and biotechnology companies is driving the market.
In 2025, the Pharmaceutical Manufacturing Services Segment is Expected to Dominate this Market
Based on type, the Europe pharmaceutical contract development and manufacturing market is segmented into pharmaceutical manufacturing services, drug development services, and biologics manufacturing services. In 2025, the pharmaceutical manufacturing services segment is expected to account for the largest share of the market. The large of this segment is attributed to factors such as the growing need to reduce manufacturing costs in the pharmaceutical industry, the requirement for high-quality bulk manufacturing, and the growing demand for generic drugs. According to EFPIA, in 2020, France accounted for 19.5% of generics sales in total pharmaceutical sales. The highest generic drug sales in Europe were for Austria, which accounted for 49%.
In 2025, the Large Pharmaceutical Companies Segment is Expected to Account for the Largest Share of the Market
Based on end user, the Europe pharmaceutical contract development and manufacturing market is segmented into large pharmaceutical companies, small & mid-size pharmaceutical companies, and generic pharmaceutical companies. In 2025, the large pharmaceutical companies segment is expected to account for the largest share of the market. The factors attributing to the largest share of the market are the growing need for state-of-the-art processes and production technologies, the need for business expansion globally, the growing strategic focus of sponsors to focus on core business functions such as marketing and R&D, and continued efforts of pharmaceutical companies to reduce production costs.
Furthermore, the outbreak of the COVID-19 pandemic has led CDMOs to enter into strategic partnerships or agreements for large-scale vaccine development and manufacturing. In May 2021, Siegfried Holdings AG (Switzerland) signed an agreement with Novavax, Inc. (U.S.) to manufacture and supply Novavax's protein-based COVID-19 Vaccine. Under this agreement, Siegfried served as a non-exclusive supplier for the aseptic fill and finish of Novavax's COVID-19 vaccine NVX-CoV2373.
Germany: Fastest Growing Market
Germany is expected to register the highest CAGR during the forecast period. Growing government investments in the life sciences industry and the increasing prevalence of chronic diseases are the major factors generating high demand for pharmaceuticals in the country. In addition, the shift towards developing precision medicines and high competition from generics is compelling the pharmaceutical and biotechnology industry to increase their production and outsource their activities to accelerate the development process.
Key Players
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments adopted by leading market players in the industry over the years. The key players operating in the Europe pharmaceutical contract development and manufacturing market are AbbVie Inc. (U.S.), Aenova Group (Germany), Wuxi Biologics, Inc. (China), Vetter Pharma International GmbH (Germany), Catalent, Inc. (U.S.), Lonza Group Ltd. (Switzerland), Recipharm AB (Sweden), Almac Group (U.K.), C.H. Boehringer Sohn Ag & Co. KG. (Germany), Eurofins Scientific (France), Curia Global, Inc. (U.S.), Thermo Fisher Scientific, Inc. (U.S.), Evonik Industries AG (Germany), Cambrex Corporation (U.S.), Siegfried Holdings AG (Switzerland), Fabbrica Italiana Sintetici S.p.A. (Italy), and Corden Pharma GmbH (Germany).
Scope of the Report:
Europe Pharmaceutical Contract Development and Manufacturing Market Assessment, by Type
Europe Pharmaceutical Contract Development and Manufacturing Market Assessment, by End User
Europe Pharmaceutical Contract Development and Manufacturing Market Assessment, by Country
Key questions answered in the report:
The Europe pharmaceutical contract development and manufacturing market study different types of services, such as pharmaceutical manufacturing services, drug development services, and biologics manufacturing services used by end users. The Europe pharmaceutical contract development and manufacturing market studied in this report involves the analysis of various segments at country levels.
The Europe pharmaceutical contract development and manufacturing market is projected to reach $68.12 billion by 2032 at a CAGR of 7.4% from 2025 to 2032
Among all the types studied in the report, the pharmaceutical manufacturing services segment is expected to account for the largest share of the market in 2025. The large share of the segment is attributed to the growing need to reduce manufacturing costs, bring a new drug to the market quickly at the lowest possible cost, the requirement for high-quality bulk manufacturing, and the growing demand for generic drugs.
The growth of this market is driven by the complex manufacturing requirements of the pharmaceutical industry, manufacturers' growing inclination toward the use of cutting-edge technologies, patent expiration, increasing investments in pharmaceutical R&D, and the rising demand for generic medicines & biologics. In addition, the growing demand for cell and gene therapies and personalized medicine and growth in high potency active pharmaceutical ingredients (HPAPI) and antibody-drug conjugates (ADC) markets is expected to offer significant opportunities for the growth of the pharmaceutical contract development and manufacturing market.
The key players operating in the Europe pharmaceutical contract development and manufacturing market are AbbVie Inc.(U.S.), Aenova Group (Germany), Wuxi Biologics, Inc. (China), Vetter Pharma International GmbH (Germany), Catalent, Inc. (U.S.), Lonza Group Ltd. (Switzerland), Recipharm AB (Sweden), Almac Group (U.K.), C.H. Boehringer Sohn Ag & Co. KG. (Germany), Eurofins Scientific (France), Curia Global, Inc. (U.S.), Thermo Fisher Scientific, Inc. (U.S.), Evonik Industries AG (Germany), Cambrex Corporation (U.S.), Siegfried Holdings AG (Switzerland), Fabbrica Italiana Sintetici S.p.A. (Italy), and Corden Pharma GmbH (Germany).
Germany is expected to offer significant growth opportunities owing to the government initiatives to create favorable pharmaceutical drug approval programs, increase investment from existing pharmaceutical manufacturers in the country for infrastructure development, and growing investments towards biosimilar manufacturing.
























Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates