Resources
About Us
Europe Continuous Bioprocessing Market by Product (Filtration, Chromatography, Centrifuges, Consumables), Application (Commercial {Vaccines, Monoclonal Antibodies}, R&D), End User (Pharmaceuticals, Biotechnology, CROs) - Forecast to 2032
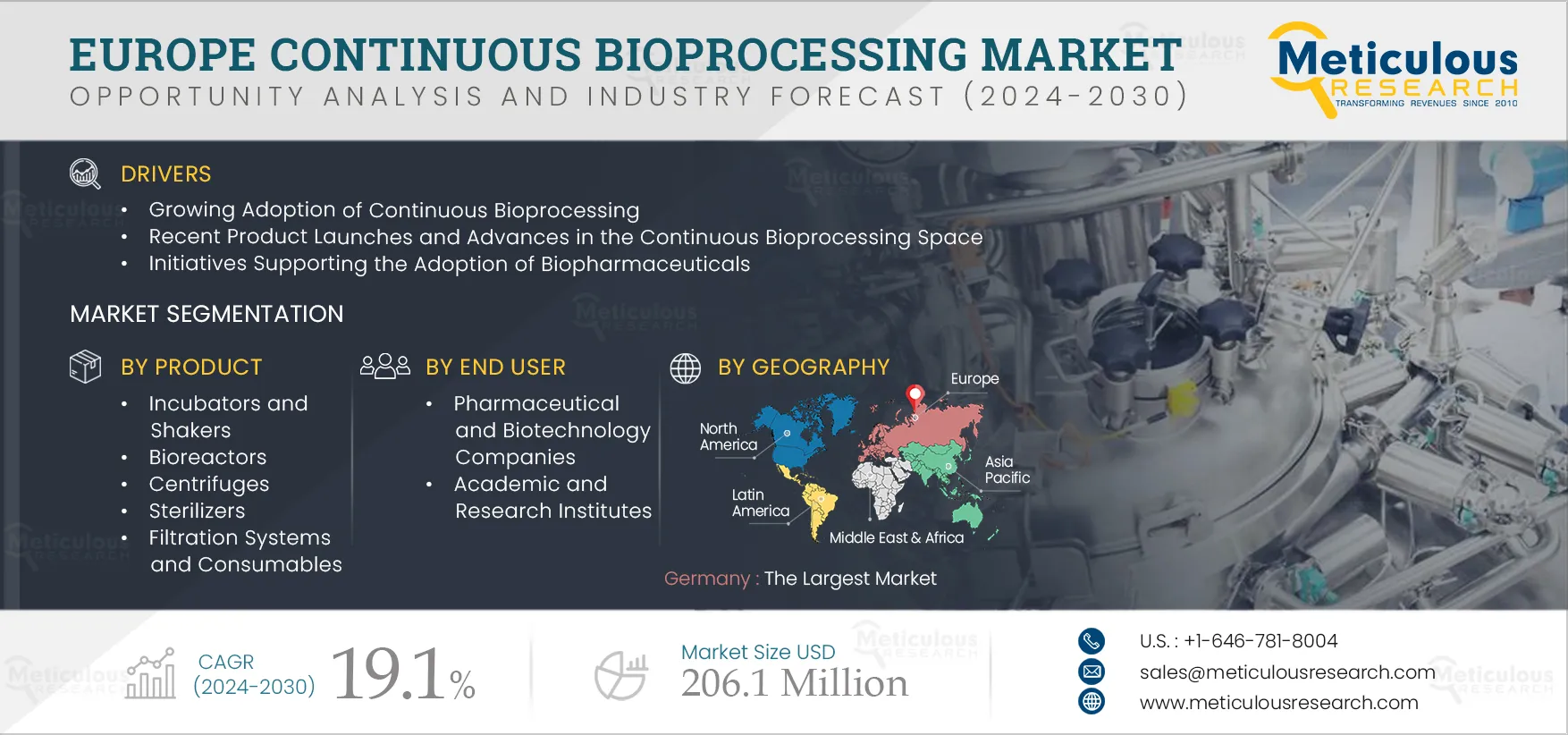
Report ID: MRHC - 104922 Pages: 110 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Europe Continuous Bioprocessing Market is expected to grow at a CAGR of 19.1% from 2025 to 2032 to reach $206.1 million by 2032. Continuous manufacturing is an emerging trend spanning various industries. From automotive to paper, businesses are embracing continuous manufacturing to enhance efficiency and bolster profits. The shift from batch to continuous manufacturing is gaining traction in the biopharmaceuticals sector due to the surge in demand for intricate therapies and heightened market competition. Continuous bioprocessing is increasingly being adopted due to its agility, flexibility, efficiency, and robustness. This approach streamlines processes by minimizing steps, utilizing compact facilities and equipment, and enhancing product quality to facilitate real-time release.
The growth of the continuous bioprocessing market in Europe is attributed to recent product launches and advances in the continuous bioprocessing space, gradual adoption of continuous manufacturing, and initiatives supporting the adoption of biopharmaceuticals. Furthermore, the shift towards bioprocessing 4.0 and the rising adoption of personalized medicines are expected to provide significant market growth opportunities.
However, the limitations of continuous bioprocessing may restrain the growth of this market. Additionally, manufacturers’ hesitation to shift from batch manufacturing to continuous manufacturing poses challenges to the market players.
Click here to: Get Free Sample Pages of this Report
Over the years, significant strides have been taken in bioprocessing to achieve cost reductions, enhance product yield, and seamlessly integrate upstream and downstream processing. Progress in upstream processing has been concentrated on essential aspects such as elevating volumetric productivity through process intensification, amplifying cell-specific productivity, leveraging cellular and molecular biology, and expediting process development timelines.
Bioprocessing operations have also led to continuous improvements in cell expression systems and the development and improvement of disposable bioreactors impacting bioprocessing operations, leading to new product launches. For instance,
Thus, the increasing product launches and innovation are driving the growth of the continuous bioprocessing market in Europe.
Capacity Expansion by Vendors Supports the Europe Continuous Bioprocessing Market
The demand for innovative manufacturing methods like continuous processing is rising due to the increasing influx of vaccine and therapeutic candidates in the pipeline. Furthermore, the impact of COVID-19 has increased the need for extensive-volume bioproduction. In response, numerous vendors are scaling up their manufacturing and production capacities to cater to the escalating demand for biopharmaceutical products. For instance,
In 2025, the filtration systems and consumables segment is expected to account for the largest share of the Europe continuous bioprocessing market
In 2025, the filtration systems and consumables segment is estimated to account for the largest share of the Europe continuous bioprocessing market. Filtration stands as a preferred purification method in the biopharmaceutical sector. The surge in advanced filtration systems and consumables, such as disposable or single-use systems, combined with the filters' reusability across various bioprocessing steps, contributes to the significant market share of this segment.
In 2025, the commercial application segment is expected to account for the largest share of the Europe continuous bioprocessing market
In 2025, the commercial application segment is estimated to account for the largest share of the Europe continuous bioprocessing market. Growing initiatives supporting the adoption of biopharmaceuticals, rising biopharmaceutical manufacturing outsourcing, growing investments in constructing facilities compatible with continuous bioprocessing for the commercial manufacture of biopharmaceuticals, and accelerated developments in personalized therapies drive the adoption of continuous bioprocessing for commercial application. For instance, in April 2021, Evotec (Germany) started the construction of its new J.POD 2 EU cGMP biomanufacturing facility in France. The new facility, Evotec’s first in Europe, will offer capacity, flexibility, and quality for biotherapeutic development and manufacturing while utilizing its subsidiary Just – Evotec Biologics’ technology that employs small, automated, highly intensified, and continuous bioprocessing operations inside cleanrooms.
In 2025, the pharmaceutical and biotechnology companies segment is expected to account for the largest share of the Europe continuous bioprocessing market
In 2025, the pharmaceutical and biotechnology companies segment is estimated to account for the largest share of the Europe continuous bioprocessing market. The large share of this segment is attributed to factors such as the transition of pharma and biopharma manufacturers from batch to continuous processing, regulatory authorities' increasing emphasis on continuous bioprocessing, and the rising investments directed towards the integration of continuous processing technologies.
Germany to account for the largest market share in 2025
In 2025, Germany is estimated to dominate the Europe Continuous Bioprocessing market. Germany’s significant market share is primarily attributed to favorable initiatives supporting the adoption of biosimilars, a strong biopharma market compared to other European countries, and the presence of leading players in Germany.
Key Players
The report includes a competitive landscape based on the key growth strategies adopted by leading market players in the past four years. The key players profiled in the Europe Continuous Bioprocessing market study are 3M Company (U.S.), Cytiva (U.S.), Thermo Fisher Scientific Inc. (U.S.), Merck KGaA (Germany), Sartorius AG (Germany), Repligen Corporation (U.S.), Eppendorf AG (Germany), Applikon Biotechnology (Netherlands), Pall Corporation (U.S.), Solida Biotech GmBH (Germany), Electrolab Biotech Limited (U.K.), Biowest (France), and Bionet (U.S.).
Scope of the Report:
Europe Continuous Bioprocessing Market Assessment, by Product
Europe Continuous Bioprocessing Market Assessment, by Application
Europe Continuous Bioprocessing Market Assessment, by End User
Europe Continuous Bioprocessing Market Assessment, by Country
Key questions answered in the report:
This report covers the market sizes & forecasts for various products, applications, end user, and geography. It also involves the value analysis of various segments and sub-segments of continuous bioprocessing at the country level.
The Europe continuous bioprocessing market is projected to reach $206.1 million by 2032, at a CAGR of 19.1% during the forecast period of 2025–2032.
Based on product, in 2025, the filtration systems and consumables segment is estimated to account for the largest share of the Europe continuous bioprocessing market.
The growth of this market is attributed to factors such as recent product launches and advances in the continuous bioprocessing space, gradual adoption of continuous manufacturing, and initiatives supporting the adoption of biopharmaceuticals. Furthermore, the shift towards bioprocessing 4.0 and the rising adoption of personalized medicines are expected to provide significant market growth opportunities.
The limitations of continuous bioprocessing may restrain the growth of this market.
The key players operating in the Europe continuous bioprocessing market are 3M Company (U.S.), Cytiva (U.S.), Thermo Fisher Scientific Inc. (U.S.), Merck KGaA (Germany), Sartorius AG (Germany), Repligen Corporation (U.S.), Eppendorf AG (Germany), Applikon Biotechnology (Netherlands), Pall Corporation (U.S.), Solida Biotech GmBH (Germany), Electrolab Biotech Limited (U.K.), Biowest (France), and Bionet (U.S.).
The market in Germany is projected to register the highest growth rate during the forecast period and offer significant growth opportunities for the players operating in this market. The presence of key market players and rising government initiatives aimed at promoting the adoption of biosimilars are key factors driving the market’s growth in Germany.
























Published Date: Jan-2025
Published Date: Mar-2024
Published Date: Jan-2024
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates