Resources
About Us
Endotherapy Devices Market by Type (Needles, Biopsy Forceps, Balloon Dilators, Haemostasias, Snares, Catheters, Sphincterotomes, Guide Wires, Stents, Retrieval Net), Application (Upper GI Endoscopy, Colonoscopy, Cystoscopy) - Global Forecast to 2032
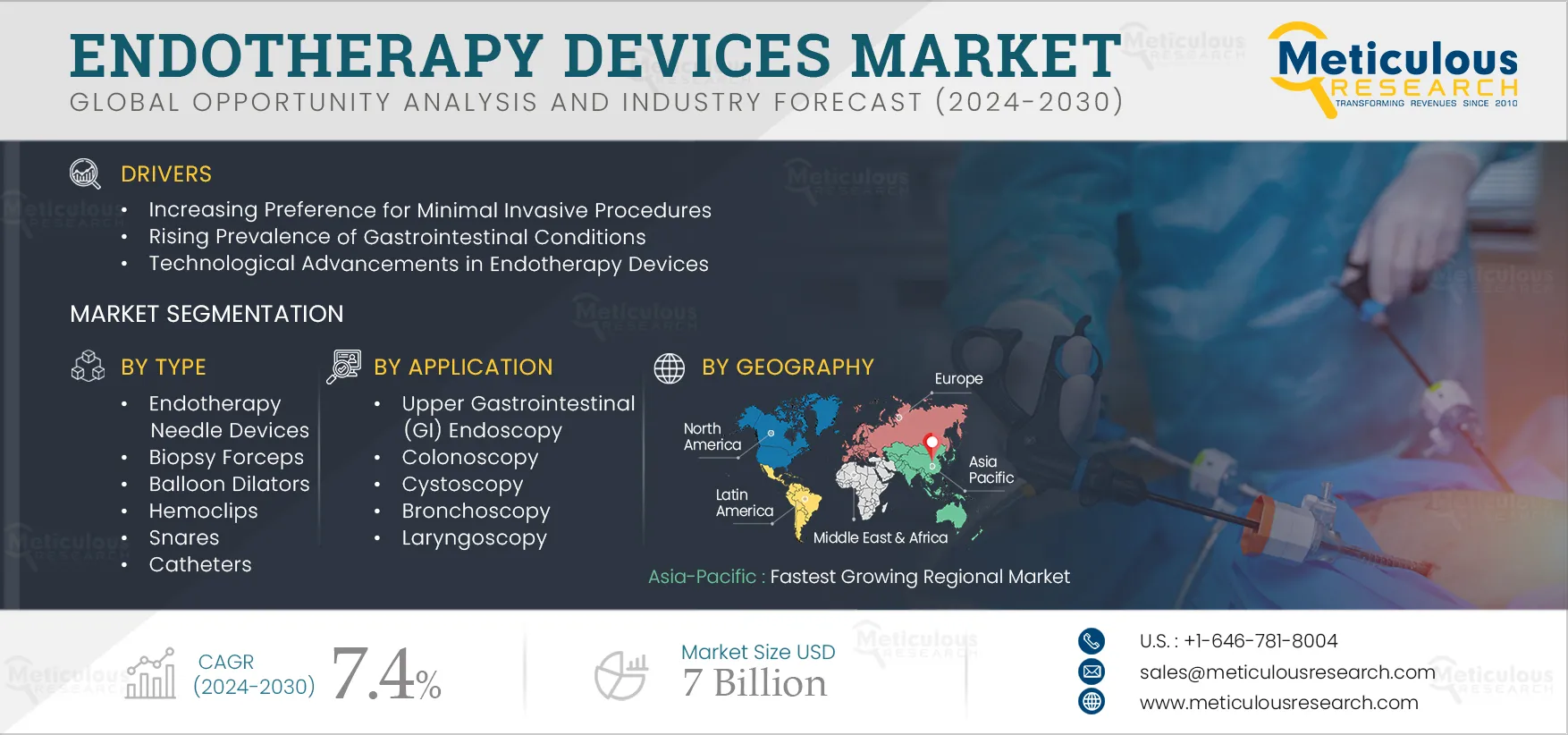
Report ID: MRHC - 104845 Pages: 210 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Endotherapy Devices Market is expected to reach $7 billion by 2032 at a CAGR of 7.4% from 2025 to 2032. Endotherapy devices are inserted in the body during endoscopic procedures. Endotherapy devices such as needles, dilators, hemostasis systems, hemoclips, stents, and extraction systems are used during colonoscopies, gastroscopies, bronchoscopies, and other procedures. Endotherapy equipment is also used to diagnose abnormal growth in the colon and other abdominal organs and digestive tract hemorrhage. Additionally, they are employed therapeutically to eliminate tumors or polyps, halt bleeding, and widen strictures or confined bodily channels.
The growth of this market is driven by the increasing preference for minimally invasive procedures, the rising prevalence of gastrointestinal conditions, and technological advancements in endotherapy devices. However, the high cost of endoscopy procedures is restraining the market. The increasing demand for endoscopic procedures in emerging markets is creating opportunities for key players in the market. Additionally, the lack of skilled professionals is a major challenge for market growth.
Click here to: Get a Free Sample Copy of this report
Medical conditions such as abnormal growth in the gastrointestinal tract, abnormal bleeding in the digestive tract, inflammatory bowel disease (IBD), ulcerative colitis (UC), and chronic pancreatitis burden the healthcare system worldwide. According to the study published by American Gastroenterological Association in 2021, about 40% of the global population suffers from functional gastrointestinal disorders. Factors such as lifestyle changes, sedentary lifestyle, diet change, stress, and growing age contribute to the increasing prevalence of gastrointestinal disorders. Endotherapy devices are used in surgical procedures to examine or remove abnormal growth in the gastrointestinal or abdominal tract.
Endotherapy devices are used in the various endoscopic procedure employed in the treatment of gastrointestinal conditions, such as endoscopic retrograde cholangiopancreatography (ERCP), endoscopic submucosal dissection (ESD), and peroral endoscopic myotomy (POEM). Endotherapy devices are used to treat narrowed esophagus, abdominal pain, bleeding in the gastrointestinal tract, removal of foreign objects, and reduce the risk of infection.
The prevalence of gastrointestinal cancer is rising across the globe. According to WHO, gastrointestinal cancer accounts for one in four cases and one in three cancer deaths each year. Endotherapy devices play a major role in collecting tissue samples from different positions of the gastrointestinal tract for biopsy. Thus, rising incidences of gastrointestinal conditions and increasing adoption of endotherapy devices for various GI disorders drive market growth.
New Product Launch and Preference for Minimally Invasive Procedure to Drive the Demand for Endotherapy Devices
The market players are developing and launching new endotherapy devices to improve clinical outcomes. For instance, in April 2021, Argon Medical Devices, Inc. launched a Halo Single-Loop Snare Kits. The kits are used in the removal of foreign objects. Earlier, in June 2020, Cook Medical (U.S.) launched a transluminal Biliary Biopsy Forceps Set (BBFS) and a new 40-cm delivery system line extension of the existing Silver 635 biliary stent (ZIB). The forceps are used for accurate tissue samples for biopsy and ZIB stent is developed for treating biliary neoplasm.
Patients are increasingly preferring endoscopic procedures over traditional open surgeries. Unlike traditional surgeries, endoscopic procedures do not require large incisions on the body, thus reducing hospital stays and associated costs. Healthcare providers also opt for endoscopy as it involves less damage to patients’ bodies. The increasing adoption of endoscopic procedures for reduced post-surgery complications, rapid recovery, increased safety than traditional surgeries, minimal pain, and reduced in-hospital stay drives the preference for endoscopy procedures and the market growth.
The Significance of Hemostasis Systems is Rising for Bleeding Control
Based on type, the global endotherapy devices market is segmented into endotherapy needles devices, biopsy forceps, balloon dilators, hemostasis systems, hemoclips, snares, catheters, sphincterotomes, guide wires, extraction balloons, extraction baskets, stents, distal caps, polyp retrieval traps, retrieval net, and other devices. The demand for hemostasis systems is expected to increase. The hemostasis system helps in stopping bleeding during the endoscopy procedure lowering the need for prolonged hospitalization and blood transfusions. The rising number of endoscopic procedures and increasing incidences of bleeding during endoscopic procedures is driving the adoption of the hemostasis system. In September 2022, Medtronic received FDA approval for the Nexpowder Endoscopic Hemostasis System. It is a hemostasis powder used to manage bleeding during endoscopy. Such technological advancement is expected to support market growth.
In 2025, the Upper Gastrointestinal (GI) Endoscopy Segment is Expected to Account for the Largest Share of the Market
Based on application, the global endotherapy devices market is segmented into upper gastrointestinal (GI) endoscopy, colonoscopy, cystoscopy, bronchoscopy, laryngoscopy, and other applications. In 2025, the upper GI endoscopy segment is expected to account for the largest share of the market. The use of endotherapy devices in upper gastrointestinal endoscopy for treating diseases such as stomach ulcers, gastritis, celiac diseases, and cancer and the high preference for minimally invasive surgeries contribute to the largest market share. The preference for minimally invasive procedures is rising among healthcare professionals and patients. The high reliability and accuracy, reduced hospital stays, and cost reduction drive the market. Additionally, the growing prevalence of GI cancer contributes to a large market share of this segment.
Asia-Pacific: Fastest Growing Regional Market
Asia-Pacific is expected to register the highest CAGR during the forecast period. The growth of the endotherapy devices market in Asia-Pacific is attributed to the advancing healthcare infrastructure, rising cases of diseases requiring endoscopies, and the growing burden of the geriatric population and associated chronic diseases. Additionally, increasing awareness about minimally invasive endoscopic procedures, emerging medical tourism, and increasing healthcare spending is driving the market in the Asia-Pacific region.
Key Players
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments adopted by leading market players in the industry over the years. The key players operating in the global endotherapy devices market are Boston Scientific Corporation (U.S.), Olympus Corporation (Japan), Medtronic plc (U.S.), FUJIFILM Holdings Corporation (Japan), Cook Group Incorporated (U.S.), Conmed Corporation (U.S.), Hoya Corporation (Japan), Stryker Corporation (U.S.), KARL STORZ SE & Co. KG (Germany), Smith & Nephew PLC (U.K.), Micro-Tech (Nanjing) Co., Ltd. (China), Nipro Corporation (Japan), MEDIVATORS B.V. (Germany), The Cooper Companies, Inc. (U.S.), and JOHNSON & JOHNSON (U.S.)
Scope of the Report:
Endotherapy Devices Market Assessment, by Type
Endotherapy Devices Market Assessment, by Application
Endotherapy Devices Market Assessment, by Geography
Key questions answered in the report:
The global endotherapy devices market studies different endotherapy devices, such as needle systems, hemostasis systems, and hemoclips used for endoscopic procedures. The global endotherapy devices market studied in this report involves the analysis of various segments of endotherapy devices at regional and country levels.
The global endotherapy devices market is projected to reach $7 billion by 2032 at a CAGR of 7.4% from 2025 to 2032
Based on application, the upper gastrointestinal (GI) endoscopy segment is estimated to hold the largest market share in 2025. Factors such as the high prevalence of target disorders and high preference for minimally-invasive procedures support the largest share of the market.
The growth of this market is driven by the increasing preference for minimally-invasive procedures, the rising prevalence of gastrointestinal conditions, and technological advancements in endotherapy devices. Increasing demand for endoscopic procedures in emerging markets is creating opportunities for key players in the market.
The key players operating in the global endotherapy devices market are Boston Scientific Corporation (U.S.), Olympus Corporation (Japan), Medtronic plc (U.S.), FUJIFILM Holdings Corporation (Japan), Cook Group Incorporated (U.S.), Conmed Corporation (U.S.), Hoya Corporation (Japan), Stryker Corporation (U.S.), KARL STORZ SE & Co. KG (Germany), Smith & Nephew PLC (U.K.), Micro-Tech (Nanjing) Co., Ltd. (China), Nipro Corporation (Japan), MEDIVATORS B.V. (Germany), The Cooper Companies, Inc. (U.S.), and JOHNSON & JOHNSON (U.S.)
The emerging countries in Asian-Pacific are projected to offer significant growth opportunities for the vendors in this market due to the advancing healthcare infrastructure, growing geriatric population, and the associated increase in the burden of chronic diseases.
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Nov-2024
Published Date: Jun-2024
Published Date: Jun-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates