Resources
About Us
eClinical Solutions Market Size, Share, Forecast, & Trends Analysis by Product (CDMS, CTMS, eCOA, ePRO, eDC, eTMF, Clinical Analytics, Safety Solutions), End User (Pharmaceutical, Clinical Research Organization, Medical Device), Phase – Global Forecast to 2032
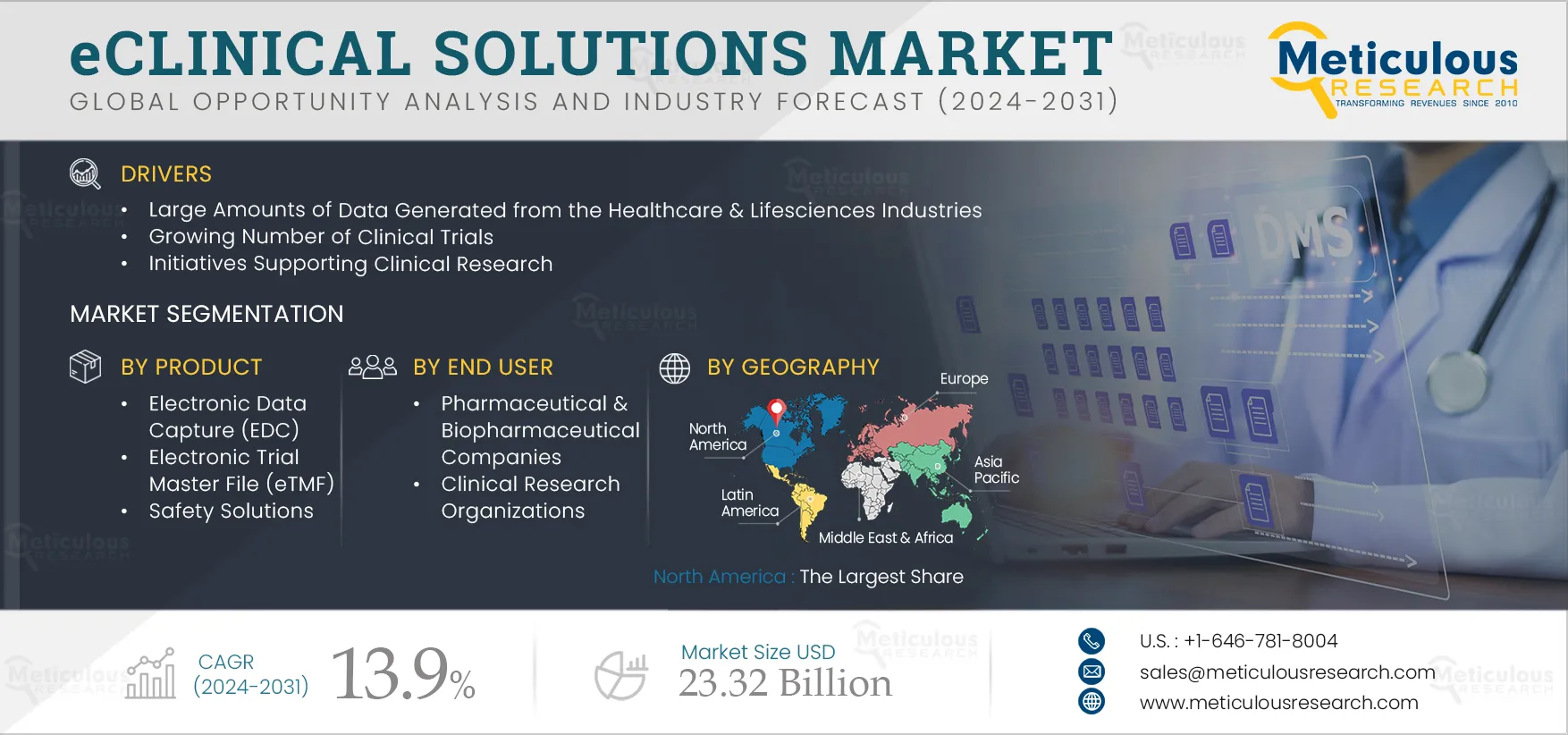
Report ID: MRHC - 1041227 Pages: 260 Jun-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe eClinical Solutions Market is expected to reach $23.32 billion by 2032, at a CAGR of 13.9% from 2025 to 2032. The growth of the eClinical solutions market is primarily driven by the large amounts of data generated by the healthcare & life sciences industries, the growing number of clinical trials, initiatives supporting clinical research, and increasing funding for medical & pharmaceutical research & development. Moreover, IT infrastructure strengthening initiatives by large hospital chains, and the rising demand for precision medicine are expected to generate growth opportunities for the players operating in the eClinical solutions market.
The healthcare industry generates a large amount of data, which is expected to reach 10,000 exabytes by 2025. This data is generated by hospitals, clinics, patients, wearables, and sensors. The healthcare industry generates about 30% of the world's data, and its compound annual growth rate is expected to reach 36% by 2025. The healthcare industry generates petabytes of data each year from medical devices, health imaging, and EHRs. Hospitals alone generate 50 petabytes of data annually. This data can be used for clinical trials to determine genetics and other factors. eClinical solutions can help organize, manage, and structure this data to improve the healthcare industry and simplify data management and analytics for clinical trials.
 Click here to: Get Free Sample Pages of this Report
Click here to: Get Free Sample Pages of this Report
AI and ML technologies have proved beneficial for the healthcare industry. In recent years, the healthcare industry has seen growth in the application of AI and ML, particularly in clinical data analysis, improving the quality of patient care and clinical research. By utilizing machine learning algorithms and artificial intelligence data management tools, medical researchers and healthcare organizations can streamline clinical data processes, enhance data accuracy, and secure sensitive health information. Additionally, AI and ML-based eClinical solutions are used to collect data from multiple sources, including EHRs.
A Decentralized Clinical Trial (DCT) is a non-traditional clinical trial model that allows some or all of the trial's activities to take place at locations other than traditional clinical trial sites. These alternate locations can include the participants’ homes, local healthcare facilities, or nearby laboratories. eClinical solutions can be used to help sponsors navigate the complexities of managing data in decentralized clinical trials. eClinical solutions can help with protocol configuration, patient visit management, protocol amendment management, data collaboration, and trial timeline acceleration.
Precision medicine is an emerging approach to patient care. Physicians choose a treatment method based on the patient’s genetic makeup, also considering genetic changes resulting from disease and lifestyle habits. It is an emerging disease treatment and prevention approach that considers variability in genes, environments, and lifestyles among individuals.
Precision medicine requires new approaches to clinical trial design and analysis. Precision medicine trials are more complex than traditional clinical trials, which randomly assign treatments to patients who meet specific eligibility criteria. Precision medicine trials use molecular profiling to customize treatments for patients. The growing demand for personalized medicine is expected to lead to the development of new drugs requiring clinical trials for their approval, generating opportunities for the players operating in the eClinical solutions market.
Based on product, the eClinical solutions market is segmented into clinical data management systems (CDMS), clinical trial management systems (CTMS), randomization & trial supply management systems, electronic data capture (EDC), electronic clinical outcome assessments (eCOA), electronic patient-reported outcomes (ePRO), clinical analytics platforms, electronic trial master file (ETMF), clinical data integration platforms, safety solutions, and other product types. In 2025, the CDMS segment is expected to account for the largest share of 28.3% of the eClinical solutions market. The large market share of this segment is attributed to the increasing need to sort and manage the huge amount of data generated during clinical trials to gain valuable insights, and the large number of clinical trials performed each year. For instance, in 2022, 12,337 clinical trials were conducted in the Western Pacific, 13,066 in Europe, 11,935 in the Americas, 11,030 in Southeast Asia, 4,593 in the Eastern Mediterranean, and 861 in Africa (Source: World Health Organization).
However, the CTMS segment is projected to register the highest growth rate of 13.5% during the forecast period 2025–2032. The segment’s growth is driven by the increase in clinical research and clinical trials, the growing demand for organized clinical trial data, and the shift toward decentralized clinical trials.
Based on clinical trial phase, the eClinical solutions market is segmented into Phase I, Phase II, Phase III, and Phase IV. In 2025, the Phase III segment is expected to account for the largest share of the eClinical solutions market. The segment’s large share can be attributed to the large number of participants in Phase III clinical trials, the importance of Phase III clinical trials for drug approval, and the double-blind nature of Phase III clinical trials.
Phase III trials are generally double-blind, wherein neither the investigator nor the participant knows which medication is being administered. This is done to reduce or eliminate bias at the time of results interpretation. This creates a need for eClinical solutions to effectively keep a record of which medication has been administered to the participants, as the participant pool is larger in Phase III trials.
However, the Phase IV segment is expected to register the highest CAGR during the forecast period 2025-2032. The growth of this segment is attributed to the increasing importance of post-market surveillance, the growing use of real-world data and real-world evidence for the post-market surveillance of drugs, the growing number of drug approvals, and increasing R&D investments from pharmaceutical and biopharmaceutical companies.
Based on end user, the eClinical solutions market is segmented into pharmaceutical & biopharmaceutical companies, clinical research organizations, medical device manufacturers, and other end users. In 2025, the pharmaceutical & biopharmaceutical companies segment is expected to account for the largest share of the eClinical solutions market. High investments in the pharmaceutical & biopharmaceutical sectors, companies’ high focus on drug development and approvals, the high number of clinical trials conducted globally, and the need for quick and effective management of clinical trial data contribute to the segment’s large market share. Additionally, the need to extract information from clinical trials for submission to drug approval authorities also contributes to the segment’s large share.
However, the clinical research organizations segment is expected to register the highest CAGR during the forecast period. This segment’s growth is driven by pharmaceutical & biopharmaceutical companies’ increased outsourcing to clinical research organizations, the companies’ increasing focus on R&D for drug discovery and development, and the adoption of advanced technologies among CROs for clinical trial management.
In 2025, North America is expected to account for the largest share of 43.2% of the eClinical solutions market. Also, in 2025, the U.S. is expected to dominate the eClinical solutions market in North America, mainly due to the high number of clinical trials carried out in the country and stringent regulations for drug approvals.
However, Asia-Pacific is slated to register the highest CAGR of 15.2% during the forecast period, mainly due to the growing pharmaceutical & biopharmaceutical industries, the high number of clinical trials, the growing preference for eClinical solutions over manual data management and data entry procedures and increasing pharmaceutical R&D investments in countries such as India and China. Additionally, government initiatives supporting the development of new drugs also contribute to the growth of the eClinical solutions market in APAC. For instance, in September 2024, the Government of India launched the National Policy on Research and Development and Innovation in the Pharma-MedTech Sector. This policy aims to promote research and development in the pharmaceutical and med-tech sectors. Such policies are expected to strengthen the pharmaceutical sector in APAC, driving market growth.
The report offers a competitive analysis based on an extensive assessment of the leading players’ product portfolios, geographic presence, and key growth strategies adopted in the last 3–4 years. The key players operating in the eClinical solutions market are Dassault Systems S.E. (France), Fountayn (U.S.), Merative L.P. (U.S.), eClinical Solutions LLC (U.S.), Clario (U.S.), eClinicalWorks, LLC (U.S.), IQVIA Inc. (U.S.), Parexel International (MA) Corporation (U.S.), MaxisIT Inc. (U.S.), Signant Health (U.S.), Castor (U.S.), Veeva Systems Inc. (U.S.), and Oracle Corporation (U.S.).
In September 2024, Data Cubed, LLC (U.S.) launched an eClinical platform app in China.
In June 2024, ATCOR Medical (China) partnered with Data Cubed, LLC (U.S.) to jointly conduct trial monitoring across therapeutic programs.
|
Particulars |
Details |
|
No. of Pages |
260 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2024 |
|
CAGR |
13.9% |
|
Market Size (Value) |
$23.32 Billion by 2032 |
|
Segments Covered |
By Product
By Clinical Trial Phase
By End User
|
|
Countries Covered |
North America (U.S. and Canada), Europe (Germany, France, U.K., Spain, Italy, Switzerland, Sweden, Netherlands, and Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, Australia, Singapore, and Rest of APAC), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East & Africa |
|
Key Companies Profiled |
Dassault Systems S.E. (France), Fountayn (U.S.), Merative L.P. (U.S.), eClinical Solutions LLC (U.S.), Clario (U.S.), eClinicalWorks, LLC (U.S.), IQVIA Inc. (U.S.), Parexel International (MA) Corporation (U.S.), MaxisIT Inc. (U.S.), Signant Health (U.S.), Castor (U.S.), Veeva Systems Inc. (U.S.), and Oracle Corporation (U.S.) |
The eClinical solutions market report covers market sizes & forecasts based on product, clinical trials phase, and end user. This report includes a value analysis of various segments and subsegments of the eClinical solutions market at the regional and country levels.
The eClinical solutions market is projected to reach $23.32 billion by 2032, at a CAGR of 13.9% during the forecast period.
In 2025, the Clinical Data Management Systems (CDMS) segment is expected to account for the largest share of the eClinical solutions market.
The growth of the eClinical solutions market is primarily driven by the large amounts of data generated by the healthcare & life sciences industries, the growing number of clinical trials, initiatives supporting clinical research, and increasing funding for medical & pharmaceutical research & development. Moreover, IT infrastructure strengthening initiatives by large hospital chains, and the rising demand for precision medicine are expected to generate growth opportunities for the players operating in the eClinical solutions market.
The key players operating in the eClinical solutions market are Dassault Systems S.E. (France), Fountayn (U.S.), Merative L.P. (U.S.), eClinical Solutions LLC (U.S.), Clario (U.S.), eClinicalWorks, LLC (U.S.), IQVIA Inc. (U.S.), Parexel International (MA) Corporation (U.S.), MaxisIT Inc. (U.S.), Signant Health (U.S.), Castor (U.S.), Veeva Systems Inc. (U.S.), and Oracle Corporation (U.S.).
APAC countries, such as India and China, are projected to offer significant growth opportunities for the vendors in this market due to growth in the pharmaceutical and biopharmaceutical industries, the growing number of clinical trials, increasing preference for eClinical solutions over manual data management and data entry procedures, rising pharmaceutical R&D investments, and favorable government initiatives.
























Published Date: Jan-2025
Published Date: Jun-2024
Published Date: Oct-2022
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates