Resources
About Us
Digital Biomanufacturing Market Size, Share, Forecast, & Trends Analysis by Offering (Software [PAT, MES, Digital Twin] Hardware), Functionality (Product Design, Analytics, Automation), Application (MAB, Protein, Vaccine), and Bioprocess - Global Forecast to 2032
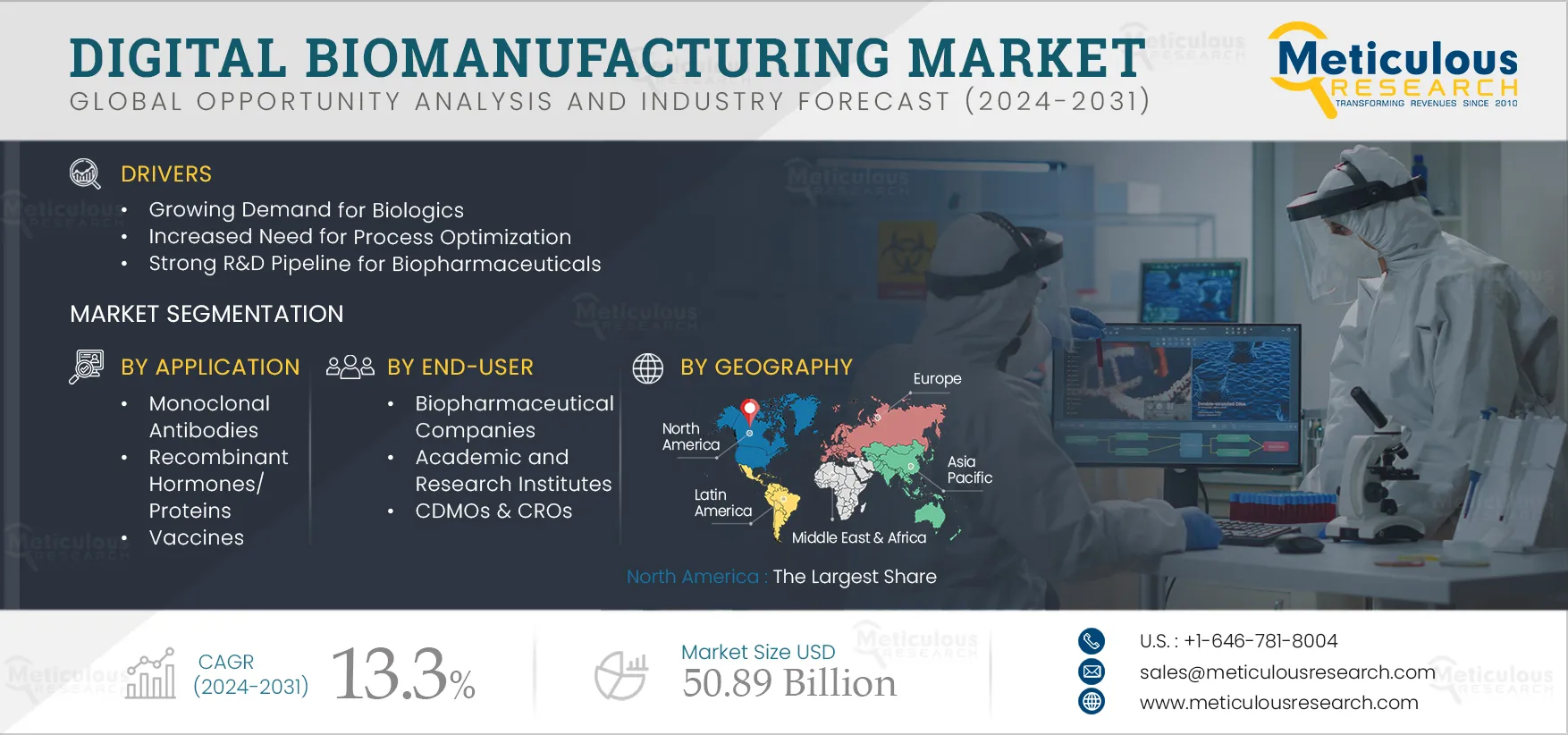
Report ID: MRHC - 1041288 Pages: 300 Sep-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Digital Biomanufacturing Market is projected to reach $50.89 billion by 2032 at a CAGR of 13.3% from 2025 to 2032. The growth of this market is attributed to various factors such as growing demand for biologics, capacity expansions of biopharmaceutical plants by key players, increased need for process optimization, growing focus on quality standards & regulatory compliance, strong R&D pipeline for biopharmaceuticals, and rising emphasis on continuous bioprocessing. Additionally, the introduction of Industry 4.0, availability of advanced data analysis tools, and shift towards bioprocessing 4.0 are expected to offer growth opportunities.
Process optimization involves improving manufacturing by incorporating quality monitoring, machinery, and maintenance, among other factors, to optimize costs and increase productivity. The biomanufacturing process is enhanced by process optimization through the identification and resolution of bottlenecks and the assurance of product quality. Process optimization techniques like design of experiments (DoE), statistical process control (SPC), and process analytical technology (PAT), assist biomanufacturers in reducing manufacturing costs and time for the biologics production by incorporating real time monitoring, continuous processing, and data capturing & analysis.
Biomanufacturing is an expensive method due to the need for specific culture conditions, costly media, and feed. The rising preference for biopharmaceuticals is driving the increased need for process optimization to achieve better yield and product quality. Thus, to scale up the business and to meet growing market demand biopharmaceutical manufacturers are increasing the adoption of process optimization techniques.
Click here to: Get Free Sample Pages of this Report
The adoption of continuous bioprocessing is being driven by the rising demand for biopharmaceuticals, supportive global initiatives for their adoption, and the advantages of continuous bioprocessing over traditional batch processing. Continuous bioprocessing facilities are at least 70% smaller than batch production facilities, leading to reduced operating costs. Additionally, continuous bioprocessing alleviates bottlenecks in the manufacturing process, which increases production efficiency and, consequently, lowers the price of biopharmaceuticals.
Government initiatives, supporting the adoption of biopharmaceuticals, are driving the demand for continuous bioprocessing. For instance, in September 2022, the U.S. government announced an investment of USD 2 billion in a biomanufacturing initiative aimed at expanding and improving the domestic biopharmaceutical manufacturing capacity. The benefits of continuous bioprocessing, such as shorter product development times and enhanced process design flexibility, are driving its increasing adoption.
Digital twin technology helps in predicting and optimizing maintenance for biomanufacturing process and facilities. It provides real-time visualization of products, processes, and their performances. This technology enables real-time monitoring through simulation, ensuring product quality and cost reduction by identifying defects in the ongoing manufacturing process. This enhances manufacturing planning and risk analysis.
Digital twin technology ensures that the planned process is proceeding as intended, maintaining time efficiency and process optimization through the implementation of effective strategies. For instance, Dassault Systèmes SE (France) offers a Virtual Twin for the biomanufacturing industry. The product provides a digital replica of the pharmaceutical process to optimize and predict the biomanufacturing process. It also helps in the resource planning, building manufacturing plants, simulating & analyzing production scenarios, and unify manufacturing operations.
Integration of advanced technologies like AI & ML improves monitoring and controlling of the entire manufacturing process. AI & ML assist in drug discovery, design & development, as well as planning and monitoring product quality. They enhance process optimization and resource management throughout the process. AI helps in structuring data and workflow of the machines & equipment involved in the manufacturing process.
ML helps in various processes like design and planning of biologics, connecting it to actual manufacturing process, and increasing efficiency of working models. For instance, in July 2022, EMBL-European Bioinformatics Institute (EBI), a part of the European Molecular Biology Laboratory developed AlphaFold, AI system in collaboration with Google DeepMind to provide predictive 3D protein structures. This helps in forecasting of upcoming activities, risk analysis, decision making, and being preemptive for further execution.
The biopharmaceutical industry is shifting towards Bioprocessing 4.0 (a term derived from Industry 4.0), a national strategic initiative launched by the German government in 2010. Biomanufacturing 4.0 is still in its initial stage. Bioprocessing 4.0 is defined as an end-to-end connected bioprocess, where all equipment and systems in the process are connected digitally through the industrial-internet-of-things (IIoT) to run, control, and enhance processes through feedback loops, machine learning, and artificial intelligence (AI). Digital transformation has become necessary in the biopharmaceutical industry as it provides the competitive advantage and opportunity for streamlining the production process. Digital biomanufacturing uses IoT to connect different data sources, equipment, and materials for process optimization. In combination with digital biomanufacturing solutions the adoption of bioprocessing 4.0 is gradually increasing. Many biopharmaceutical manufactures are shifting towards bioprocessing 4.0 to reduce the manual intervention during drug testing and monitoring which saves time and reduces the risk of errors and cross-contamination, thus boosting production.
Based on offering, the digital biomanufacturing market is segmented into software and hardware. In 2025, the software segment is expected to account for the largest share of 74.4% of digital biomanufacturing market. Software used in bioprocessing such as manufacturing execution systems (MES), process analytical technology (PAT), digital twin and data analysis software (DAS) help in designing and planning of drug or biologics from development to manufacturing. The software also provides process monitoring and control with simulation techniques, predictive outcomes, risk analysis, and decision making. The usage of software in the entire biomanufacturing process is contributing to the largest share of the market.
Additionally, the rising demand of biologics and recent approvals for biologics, increasing R&D activities across biotechnology industry, increasing funding for biopharmaceutical development are supporting the largest share of software.
Based on functionality, the digital biomanufacturing market is segmented into product design, process optimization & analytics, automation & control, and other applications. In 2025, the process optimization & analytics segment is expected to account for the largest share of digital biomanufacturing market. Process optimization and analytics technology help biomanufacturing companies in removing halts during the manufacturing process by controlling the parameters such as pH, temperature, and others which affect final quality of product. Process optimization functionalities tools have proven to be beneficial for the biomanufacturers for increasing efficiency and improving product quality, reducing waste, increasing flexibility and profitability. Thus, the adoption of process optimization & analytics solutions is highest among end-users.
Based on bioprocess, the digital biomanufacturing market is segmented into upstream bioprocess and downstream bioprocess. In 2025, the downstream bioprocess segment is expected to account for the largest share of the digital biomanufacturing market. Downstream bioprocesses are complex and time-consuming activities which delays the product to reach the market. Also, downstream processing is considered as a crucial step for the recovery and purification of biologics. Recovery process can be affected by change in temperature and pH, hence digital biomanufacturing solutions are employed for the real-time monitoring of downstream process. Additionally, digital biomanufacturing solutions provide predictive control over the downstream process by calculating process deviations based on gathered data. Thus, it helps maintaining the stability of the process and accelerates production, hence the demand for digital biomanufacturing in downstream bioprocess is increasing.
Based on application, the digital biomanufacturing market is segmented into monoclonal antibodies, recombinant hormones/proteins, vaccines, cellular-based biologics, and gene-based biologics. In 2025, the monoclonal antibodies segment is expected to account for the largest share of 48.6% of the digital biomanufacturing market. Increasing funding for monoclonal antibody development, increasing approvals for monoclonal antibody, and increasing production capacity of monoclonal antibody are contributing to the largest share of the segment. The diagnostic use of monoclonal antibody has grown over the years owing to the rising number of approvals and increasing manufacturing capacity, and is further expected to increase with the introduction of Bioprocessing 4.0. As Bioprocessing 4.0 uses digital biomanufacturing solutions to run, control, and enhance processes, the manufacturing of monoclonal antibody is expected to increase.
Based on end-user, the digital biomanufacturing market is segmented into biopharmaceutical companies, academic and research institutes, and CDMO & CROs. In 2025, the biopharmaceutical companies segment is expected to account for the largest share of digital biomanufacturing market. The increasing preference for biopharmaceuticals, increasing research in personalized medicines, and supportive initiatives for the adoption of biopharmaceuticals are supporting the segment’s largest market share. Biopharmaceuticals are typically complex and costlier than the conventional drugs to manufacture. To reduce the errors in manufacturing and scale-up production process, biomanufacturing industries are deploying digital solutions to increase the production of biologics. The increasing number of biopharmaceutical companies along with gradually increasing adoption of digital technologies in biopharmaceutical companies is contributing to the largest share of the segment. For instance, around 55% of companies incorporated digital technologies in the workflow. (Source: Global Pharma Tek, U.S.)
In 2025, North America is expected to account for the largest share of digital biomanufacturing market. The largest share of the region is attributed to the developed healthcare infrastructure, adoption of advanced technologies, and increased investment in biopharmaceutical industry. The presence of key biopharmaceutical companies, contract development, manufacturing firms, and supportive initiatives by government are the key factor contributing to the share of digital biomanufacturing market in North America. Many of the key players are increasing the production capacity of biopharmaceuticals, thus increasing the demand for digital biomanufacturing solutions. For instance, in February 2025 Moderna, Inc. (U.S.), a pharmaceutical and biotechnology company in collaboration with the government of Canada completed the construction of new state-of-the-art mRNA vaccine production facility in Laval, Canada.
Moreover, the market in Asia-Pacific is slated to register the highest growth rate of 15.1% during the forecast period. The countries in Asia-Pacific, including China, India, and South Korea, are projected to offer significant growth opportunities for the vendors in this market. This is attributed to factors such as the rising number of biopharmaceutical companies, growing patient population, increasing disposable income, and rising foreign direct investments (FDI) for biopharmaceutical companies. In 2015, the Government of China unveiled the Made in China (MIC) 2025 plan aimed at bolstering the country's domestic manufacturing capabilities. This initiative has led to a surge in the emergence of new Chinese biopharmaceutical companies. Besides, China has emerged as an increasingly attractive R&D and manufacturing destination for international pharmaceutical companies trying to shorten product timelines and costs. Thus, the rising investment in the region’s biopharmaceutical industry is increasing the demand for digital biomanufacturing solutions thereby driving the market growth.
The report offers a competitive landscape based on an extensive assessment of the product offerings and geographic presence of leading market players and the key growth strategies adopted by them over the past few years (2021–2025). The key players operating in the global digital biomanufacturing market are GE Healthcare Technologies Inc. (U.S.), Siemens Xcelerator (Subsidiary of Siemens) (Germany), Cytiva (Subsidiary of Danaher corporation) (U.S.), ABB Ltd. (Switzerland), Sanofi S.A. (France), Emerson Electric co. (U.S.), Honeywell International, Inc. (U.S.), SAP SE (Germany), OVO Biomanufacturing Inc. (U.S.), Schneider Electric SE (France), Dassault Systèmes (France), Oracle Corporation (U.S.), and 3M (U.S.).
|
Particulars |
Details |
|
Number of Pages |
300 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2024 |
|
CAGR |
13.3% |
|
Estimated Market Size (Value) |
$50.89 billion by 2032 |
|
Segments Covered |
By Offering
By Functionality
By Bioprocess
By Application
By End-user
|
|
Countries Covered |
North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, Denmark, Ireland, Belgium, and Rest of Europe), Asia-Pacific (China, Japan, India, Australia, South Korea, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, Rest of Latin America), and Middle East & Africa. |
|
Key Companies |
The key players operating in the global digital biomanufacturing market are GE Healthcare Technologies Inc. (U.S.), Siemens Xcelerator (subsidiary of Siemens) (Germany), Cytiva (subsidiary of Danaher Corporation) (U.S.), ABB Ltd. (Switzerland), Sanofi S.A. (France), Emerson Electric Co. (U.S.), Honeywell International, Inc. (U.S.), SAP SE (Germany), OVO Biomanufacturing. (U.S.), Schneider Electric SE (France), Dassault Systemes (France), Oracle Corporation (U.S.), and 3M (U.S.). |
The global digital biomanufacturing market report covers the qualitative and quantitative analysis of the digital biomanufacturing market. This report involves the analysis of various segments of digital biomanufacturing such as offering, functionality, bioprocess, and end-user at the regional and country level. The report also provides insights on factors impacting market growth, regulatory analysis, pricing analysis, and Porter’s five forces analysis.
The global digital biomanufacturing market is projected to reach $50.89 billion by 2032 at a CAGR of 13.3% from 2025 to 2032.
Among all the offerings studied in this report, the software segment is expected to account for the largest share of 74.4% the digital biomanufacturing market, in 2025. The rising need for process optimization in the biomanufacturing industry is increasing the demand for software. Software deployment helps in evaluating product and process quality with real time monitoring, data management, and prediction-based outcomes. It helps to streamline the process of biomanufacturing.
The growth of this market can be attributed to several factors such as, growing demand for biologics, capacity expansions of biopharmaceutical plants by key players, increased need for process optimization, increased focus on quality standards & regulatory compliance, strong R&D pipeline for biopharmaceuticals, and rising focus on continuous bioprocessing. However, the excessive cost of technological deployment, complex development, and manufacturing process of biopharmaceuticals are restraining the market growth.
The key players operating in the global digital biomanufacturing market are GE Healthcare Technologies Inc. (U.S.), Siemens Xcelerator (subsidiary of Siemens) (Germany), Cytiva (subsidiary of Danaher Corporation) (U.S.), ABB Ltd. (Switzerland), Sanofi S.A. (France), Emerson Electric co. (U.S.), Honeywell International, Inc. (U.S.), SAP SE (Germany), OVO Biomanufacturing. (U.S.), Schneider Electric SE (France), Dassault Systems (France), Oracle Corporation (U.S.), and 3M (U.S.).
The market in Asia-Pacific is slated to register the highest growth rate of 15.1% during the forecast period. The countries in Asia-Pacific, including China, India, and South Korea, are projected to offer significant growth opportunities for the vendors in this market. This is attributed to factors such as the rising number of biopharmaceutical companies, growing patient population, increasing disposable income, and rising foreign direct investments (FDI) for biopharmaceutical companies.
























Published Date: Jan-2025
Published Date: Nov-2024
Published Date: Jul-2024
Published Date: Feb-2019
Published Date: May-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates