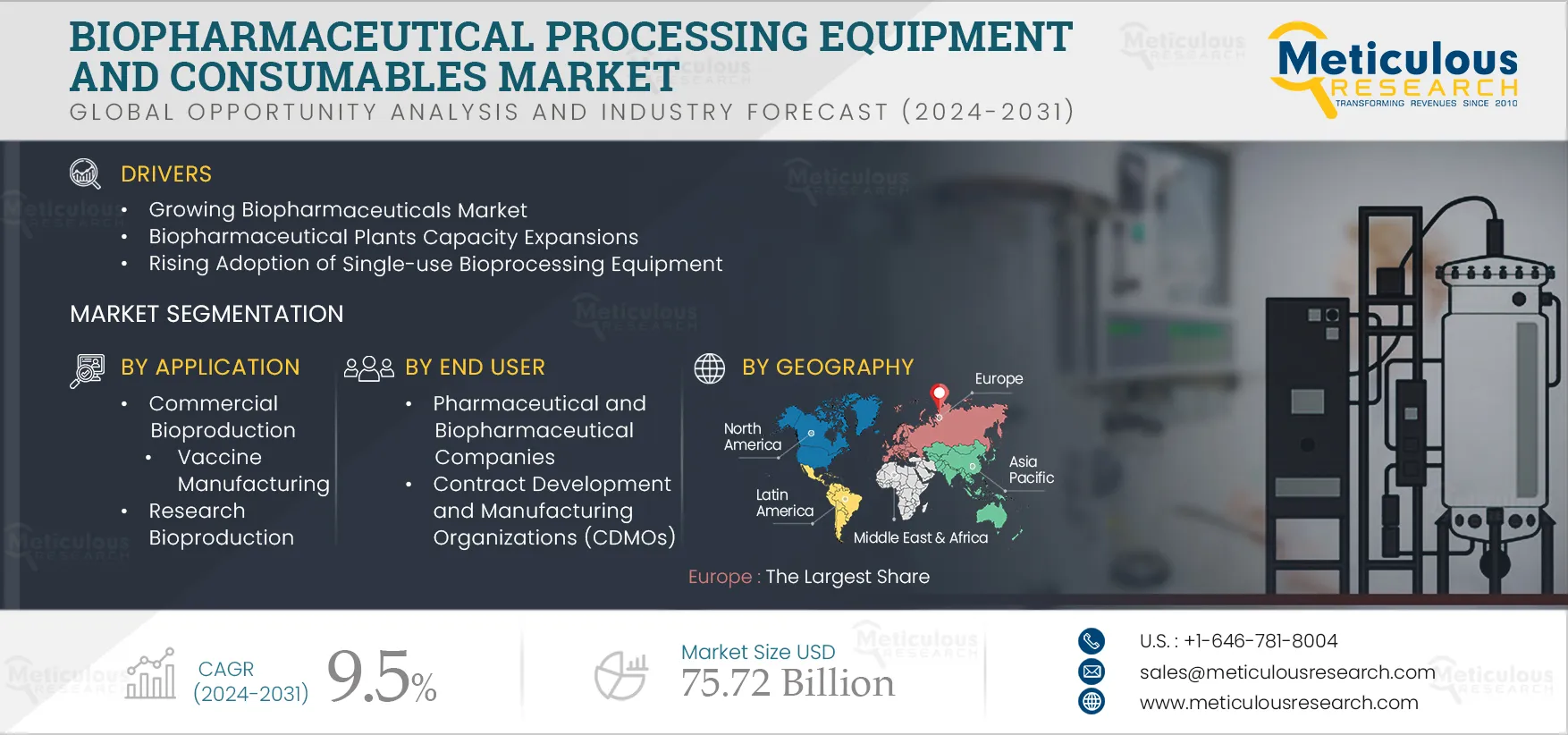
The Biopharmaceutical Processing Equipment and Consumables Market will grow at a CAGR of 9.5% during the forecast period to reach $75.72 billion by 2031. The growth of this market is driven by the growing biopharmaceuticals market, biopharmaceutical plant capacity expansions, and the rising adoption of single-use bioprocessing equipment. However, complexities in the development and manufacturing of biopharmaceuticals restrain the growth of this market. Furthermore, increasing demand for biopharmaceuticals in emerging economies, the shift toward Bioprocessing 4.0, and the rising adoption of personalized medicines are expected to generate growth opportunities for the stakeholders in this market. However, intensive capital requirements for biopharmaceutical production are a major challenge impacting market growth.
Here are the top 10 companies operating in the Biopharmaceutical Processing Equipment and Consumables Market
Thermo Fisher Scientific Inc. (U.S.)
Founded in 1956 and headquartered in Massachusetts, U.S., Thermo Fisher Scientific Inc. is a biotechnology company primarily engaged in accelerating life sciences research, solving complex analytical challenges, improving patient diagnostics, and increasing laboratory productivity. The company operates through four business segments: Life Sciences Solutions, Analytical Instruments, Specialty Diagnostics, and Laboratory Products and Services. The Life Sciences Solutions business segment is further divided into four primary businesses: Biosciences, Genetic Sciences, and BioProduction.
The company operates in the biopharmaceutical processing equipment and consumables market through its BioProduction segment. It offers chromatography and protein purification systems, cell culture products, media & consumables, cell & cell lines, and bio-reactors through its BioProduction segment.
The company’s Life Sciences Solutions business segment has a presence in the U.S. through its operational offices, laboratories, and production sites, offering services across Australia, Canada, India, China, Denmark, Finland, France, Germany, Japan, the Netherlands, Singapore, Sweden, the U.K., and the U.S. Some of the subsidiaries of the company operating in the biopharmaceutical processing equipment and consumables market are Advanced Scientifics, Inc. (ASI) (U.S.), Fisher Clinical Services (Suzhou) Co., Ltd. (China), and Thermo Fisher Germany B.V. (Netherlands).
Agilent Technologies, Inc. (U.S.)
Founded in 1999 and headquartered in California, U.S., Agilent Technologies, Inc. provides application-focused solutions for the life sciences, diagnostics, and applied chemical markets. The company operates through three business segments: Life Sciences & Applied Markets, Diagnostics & Genomics, and Agilent CrossLab. The company operates in the biopharmaceutical processing equipment and consumables market through its Life Sciences and Applied Markets business segment. It offers chromatography equipment, consumables, and components to identify, quantify, and analyze the physical and biological properties of substances and products.
The company has a presence through R&D and manufacturing sites in the U.S., Australia, China, Denmark, Germany, Italy, Japan, Malaysia, Singapore, and the U.K. With its subsidiaries and strong distribution network, it also has a presence across North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. Some of its subsidiaries operating in the biopharmaceutical processing equipment and consumables market are Agilent Technologies Luxco S.à.r.l. (Luxemburg), Agilent Technologies Singapore (Global) Pte Ltd. (Singapore) and Agilent Technologies Singapore (International) Pte. Ltd. (Singapore).
Sartorius AG (Germany)
Founded in 1870 and headquartered in Göttingen, Germany, Sartorius AG is a laboratory equipment supplier and pharmaceutical company that is involved in providing products and services for laboratory and bioprocess solutions. The company operates in two reportable segments: Bioprocess Solutions and Lab Products & Services. Bioprocess Solutions is the company’s segment that sells biopharmaceutical processing equipment and consumables. Fermenters, bioreactors, filtration and purification devices, chromatography systems, columns, and consumables are among the products available from the company. The firm has a presence in more than 20 countries worldwide. It has Bioprocess Solutions facilities in Germany, France, Puerto Rico, India, the U.K., Tunisia, Switzerland, the U.S., China, and Slovenia. Some of the company’s subsidiaries operating in the biopharmaceutical processing equipment and consumables market are Sartorius Stedim Biotech S.A. (France), Sartorius Stedim Belgium N.V. (Belgium), and WaterSep BioSeparations LLC (U.S.)
Danaher Corporation (U.S.)
Founded in 1984 and headquartered in Washington, D.C., the U.S., Danaher Corporation is a science and technology company that focuses on providing services and products for life sciences, diagnostics, and environmental sectors. The company operates through three reportable segments: Life Sciences, Diagnostics, and Biotechnology. The company operates in the biopharmaceutical processing equipment and consumables market through its Life Sciences business segment. It offers consumables and instruments for the testing and manufacturing of new drugs and vaccines.
The company is active in over 60 nations worldwide. It operates 239 facilities for administrative, sales, R&D, manufacturing, and distribution activities, with 98 in the U.S. and 141 in Europe, South America, Asia, Rest of North America, and Australia. North America, Europe, Asia, and New Zealand are home to the Life Sciences business segment’s manufacturing plants. The company’s principal subsidiaries that are involved in the biopharmaceutical processing equipment and consumables market are Beckman Coulter, Inc. (U.S.), Pall Corporation (U.S.), Phenomenex, Inc. (U.S.), and Cytiva (U.S.)
Merck KGaA (Germany)
Founded in 1668 and headquartered in Darmstadt, Germany, Merck KGaA is a science and technology company that offers products and services across therapeutic areas such as allergies, fertility, oncology, and neurodegenerative diseases. It develops drugs, diagnostic substances, and medical devices. Additionally, it provides solutions that support biotechnology and pharmaceutical research.
The company operates in three business segments: Healthcare, Life Sciences, and Performance Materials. The Life Sciences segment is further divided into three categories: Science and Lab Solutions, Process Solutions, and Life Science Services. Through its Life Sciences business segment (process solutions), it operates in the biopharmaceutical processing equipment and consumables market.
The company operates directly through its offices, subsidiaries, and facilities in countries namely, Cyprus, France, Egypt, Germany, Morocco, the Netherlands, Switzerland, Tunisia, Uruguay, Greece, China, Indonesia, South Korea, Bahrain, Lebanon, Jordan, the U.S., Iraq, Paraguay, Bolivia, Austria, Croatia, Brazil, Sweden, Romania, Macedonia, Canada, New Zealand, Singapore, Spain, Denmark, Finland, Italy, Portugal, Ireland, the U.K., Japan, Norway, Peru, and Colombia among others. It has manufacturing facilities in the U.S., Germany, and Switzerland, among others. Some of the major subsidiaries of the company are Merck Millipore Ltd. (Ireland), Millipore S.A.S. (France), Merck Serono S.A.S. (France), Merck Life Science OY (Finland), and Merck Life Science A/S (Denmark).
3M Company (U.S.)
Founded in 1929 and headquartered in Minnesota, U.S., 3M Company manufactures diverse products like adhesives, abrasives, laminates, dental products, electrical and electronic connecting and insulating materials, medical products, car-care products, electronic circuits, healthcare software, and optical films, among others. The company has four reportable business segments: Safety and Industrial, Transportation and Electronics, Healthcare, and Consumer. The healthcare business segment is further divided into sub-segments: Health Information Systems, Medical Solutions, Oral Care, Separation & Purification Sciences, and Food Safety (divested in 2022). By means of its Separation & Purification Sciences sub-segment and Healthcare business segment, the company engages in the biopharmaceutical processing, equipment, and consumables market. It provides systems for filtration and purification in the production of biopharmaceuticals.
The company offers purification filters such as activated carbon filters, membrane filters, and chromatography filters. It operates in more than 70 countries, including North America, Asia-Pacific, Europe, the Middle East, and Africa. It has a direct presence in 35 countries through its 97 manufacturing and conversion facilities.
The company has 64 manufacturing facilities in the U.S. and a strong global network of distributors. Some of its subsidiaries operating in the biopharmaceutical processing, equipment, and consumables market are 3M Purification SAS (France) and 3M Purification, Inc. (U.S.).
Bio-Rad Laboratories, Inc. (U.S.)
Founded in 1952 and headquartered in California, the U.S., Bio-Rad Laboratories, Inc. manufactures and distributes life sciences and clinical diagnostics products. The company operates in two segments: Life Sciences and Clinical Diagnostics. Through its Life Sciences business segment, it offers reagents, apparatus, and laboratory instruments in the biopharmaceutical processing equipment and consumables market. The principal end users of the company’s products are industrial research organizations, medical schools and universities, government agencies, pharmaceutical manufacturers, and biotechnology researchers.
The company operates through 140 locations in 36 countries globally; it has three R&D and manufacturing facilities for life sciences operations in the U.S., the U.K., and Singapore. The company manages its sales & administration and distribution activities across France, Switzerland, Germany, Korea, India, Thailand, Italy, the U.A.E, Japan, Australia, Belgium, Brazil, China, Denmark, Hungary, Greece, and other locations. Its major subsidiaries are Bio-Rad Laboratories (Singapore) Pte Ltd in Singapore, Bio-Rad Europe GmbH in Switzerland, and Bio-Rad Laboratories AG in Switzerland.
Eppendorf SE (Germany)
Founded in 1945 and headquartered in Hamburg, Germany, Eppendorf AG is a biotechnology company that develops, manufactures, and commercializes products & services for laboratories. The company operates in four business segments: Liquid Handling, Consumables, Separation and Instrumentation, and Lab Solutions. It operates in the biopharmaceutical processing equipment and consumables market through its Separation and Instrumentation and Lab Solutions business segments. Eppendorf offers centrifuges, shakers, mixers, bioreactors & fermenters, along with other consumables in the biopharmaceutical processing equipment and consumables market.
It operates in more than 30 countries globally, with a direct presence through its offices, sale subsidiaries, and facilities in Ireland, Belgium, the U.K., Poland, France, Austria, Denmark, Czechia, Russia, Spain, Italy, the U.A.E., India, Thailand, China, South Korea, Japan, Malaysia, the U.S., Brazil, and Australia.
Solaris Biotechnology Srl (Italy)
Founded in 2002 and headquartered in Mantovano, Italy, Solaris Biotechnology Srl manufactures equipment for biotech, pharmaceutical, food & beverage, and dairy industries. The company offers bioreactors, fermenters, cGMP solutions, and filtration systems. It also offers Clean-in-place systems, consumables, and other related instruments. In November 2021, this company was acquired by Donaldson Company, Inc. (U.S.), a provider of filtration technology and processes. Solaris Biotechnology Srl is the Life Sciences Division of Donaldson Company, Inc.
It has a direct presence in the U.S. through its subsidiary and in Malaysia through its sales & services facility. It has more than 30 distributors worldwide. Solaris Biotech USA (U.S.) is the only known subsidiary operating in the biopharmaceutical processing equipment and consumables market.
Repligen Corporation (U.S.)
Founded in 1981 and headquartered in Massachusetts, U.S., Repligen Corporation is a life sciences company that develops and commercializes bioprocessing technologies and systems to increase efficiency and flexibility in the manufacturing of biological drugs. It also provides filtration systems for biological drug manufacturing, chromatography systems for downstream processes, and protein A ligands for downstream purification for monoclonal antibody-based drugs.
The company operates 19 production facilities, subsidiaries, and offices in the U.S., Lebanon, the Netherlands, Germany, Sweden, Ireland, Estonia, France, China, India, Japan, South Korea, and Singapore. The corporation conducts business indirectly through distributors in Asia, Mexico, Russia, Armenia, Azerbaijan, Belarus, Georgia, and Kazakhstan. Some of the subsidiaries of the company operating in the biopharmaceutical processing equipment and consumables market are Repligen (Shanghai) Biotechnology Co. Ltd. (China), Repligen India Pvt. Ltd. (India), Repligen Japan LLC (Japan), Repligen Korea Co., Ltd. (South Korea), and Repligen Singapore Pte. Ltd. (Singapore).
Other Players: Microfluidics International Corporation (U.S.), Esco Micro Pte. Ltd. (Singapore), Entegris, Inc. (U.S.), GE Healthcare (U.S.), Distek, Inc. (U.S.), Boehringer Sohn AG & Co. KG (Germany), Corning Incorporated (U.S.), and Membrane Solutions (U.S.).