Resources
About Us
Asia-Pacific Point-of-Care Diagnostics Market by Application (Influenza, Pneumonia, HAI, Salmonellosis, Hepatitis, HIV, COVID, Pregnancy, Glucose Monitoring, Hematology, Tumor Marker, Urinalysis), Platform (LFA, Molecular), Sample, End User - Forecast to 2030
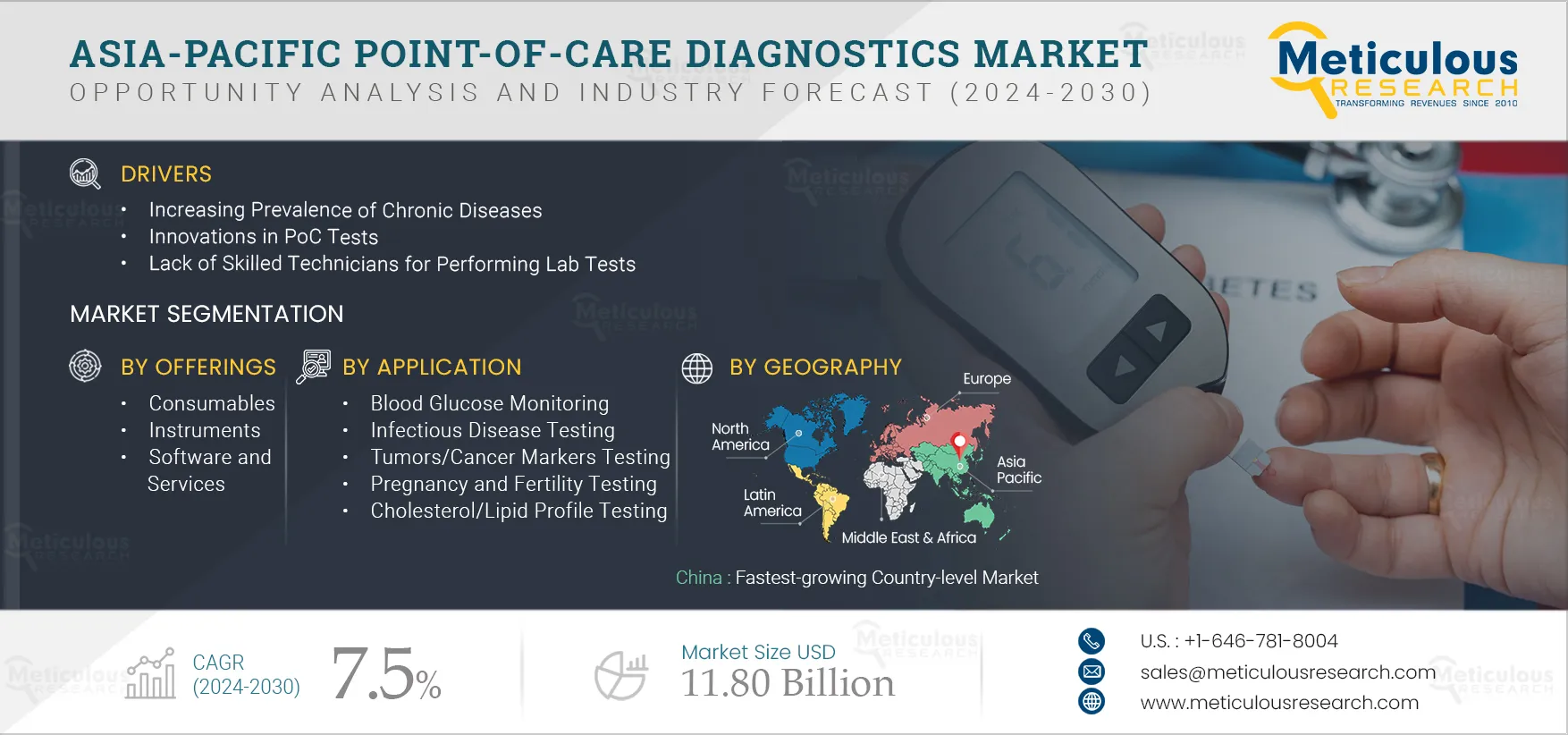
Report ID: MRHC - 104988 Pages: 200 Jul-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Asia-Pacific Point-of-Care Diagnostics Market is projected to reach $11.80 billion by 2030, at a CAGR of 7.5% from 2024 to 2030. Point-of-Care diagnostics (PoC diagnostics), also known as bedside or near-patient testing, refers to medical diagnostic testing conducted near the patient, typically at the location where healthcare is provided. The key characteristic of PoC diagnostics is the ability to deliver rapid results, often within minutes, allowing healthcare providers to make immediate treatment decisions or recommendations based on the test outcomes.
The growth of this market is driven by the increasing prevalence of chronic diseases, innovations in PoC tests, and a lack of skilled technicians for performing lab tests. However, the pricing pressure due to fluctuations in reimbursements restrains the market’s growth. Furthermore, healthcare professionals’ increasing preference for PoC tests over lab tests will offer significant market growth opportunities. However, a lack of awareness regarding the use of PoC devices challenges the market’s growth.
The demand for pathology services has grown significantly in recent years and is expected to grow further during the forecast period. However, the pathology workforce has not increased in line with this demand. Delays in initial diagnosis can lead to delays in treatment. The workforce shortage can be attributed to aging and retiring professionals and the lack of adequate histology programs to train enough new entrants.
According to Medical Futurist, by 2030, the number of active pathologists may drop by 30% compared to 2010 levels. The Chinese Pathologist Association states that there are only 20,000 licensed pathologists in China, a country with a population of over 1.4 billion. Furthermore, there is a growing need for clinical laboratory workers due to their acute shortage. Factors such as laboratory professionals retiring or leaving the profession, the increasing demand for laboratory services, and changes in clinical laboratory science practices due to technological advances contribute to this shortage of skilled professionals. This shortage disrupts the delivery of healthcare services, impacting patients and healthcare providers. Thus, the shortage of skilled professionals leads to delays in diagnosis and treatment, leading to an increased reliance on self-testing. These factors are boosting the adoption of PoC diagnostics, driving the growth of this market.
Click here to: Get Free Sample Pages of this Report
Healthcare Professionals’ Increasing Preference For PoC Tests Over Lab Tests Create an Opportunity for Market Growth
Many healthcare professionals in hospitals and clinical laboratories prefer using point-of-care diagnostics to diagnose various health conditions promptly. Healthcare professionals are replacing established diagnostic methods (microscopy, pathogen culturing, biochemical testing, conventional polymerase chain reaction (PCR), enzyme-linked immunoassay (ELISA), and other time-consuming diagnostic methods) with new point-of-care diagnostic techniques that provide higher levels of efficiency and reproducibility. As point-of-care diagnostics have superior utility, experts are changing their traditional approaches. This acceptance of point-of-care diagnostics is supporting the research & development efforts of clinical laboratories and other health-services companies in the diagnostics industry. This inclination towards point-of-care methods and practices among physicians drives the growth of the point-of-care diagnostics market.
In 2024, the Consumables Segment is Expected to Dominate the Asia-Pacific Point-of-Care Diagnostics Market
Among the offerings included in the report, in 2024, the consumables segment is expected to account for the largest share of the Asia-Pacific Point-of-Care Diagnostics Market. Factors such as the increasing number of immunoassay and molecular diagnostics tests being performed globally, leading to recurrent purchases of kits and reagents, rise in product approvals, and technological advancements in PoC testing are contributing to the large share of this market.
In 2024, the Blood Sample Segment is Expected to Dominate the Asia-Pacific Point-of-Care Diagnostics Market
Among the sample types included in the report, in 2024, the blood sample segment is expected to account for the largest share of the Asia-Pacific Point-of-Care Diagnostics Market. The large market share of this segment is attributed to the availability of a large variety of tests using blood samples, increasing prevalence of various chronic diseases, and increasing consumer awareness regarding the monitoring of chronic diseases.
In 2024, the Blood Glucose Monitoring Segment is Expected to Dominate the Asia-Pacific Point-of-Care Diagnostics Market
Among the applications included in the report, in 2024, the blood glucose monitoring segment is expected to account for the largest share of the Asia-Pacific Point-of-Care Diagnostics Market. The large market share of this segment is attributed to the rising prevalence of diabetes and the growing need for blood glucose monitoring. For instance, according to the International Diabetes Federation (IDF), in Southeast Asia, the number of adults with diabetes is estimated to reach 113 million by 2030 from 90 million in 2021.
In 2024, the Lateral Flow Assays Segment is Expected to Dominate the Asia-Pacific Point-of-Care Diagnostics Market
Among the platforms included in the report, in 2024, the lateral flow assays segment is expected to account for the largest share of the Asia-Pacific Point-of-Care Diagnostics Market. Lateral flow assays are simple one-step assays that are low-cost devices. The major factors contributing to this segment's largest share are the growing prevalence of chronic and infectious diseases, technological innovations, and the advantages of lateral flow assays, such as cost-effectiveness and rapid results.
In 2024, the Hospitals Segment is Expected to Dominate the Asia-Pacific Point-of-Care Diagnostics Market
Among the end users included in the report, in 2024, the hospitals segment is expected to account for the largest share of the Asia-Pacific Point-of-Care Diagnostics Market. The rising number of hospitals and clinics, increasing demand for point-of-care diagnostics, and increasing healthcare expenditure worldwide are driving the demand for PoC diagnostics. Furthermore, the outbreak of the COVID-19 pandemic has resulted in an increased demand for PoC diagnostic kits in hospitals & clinics for the treatment and prevention of COVID-19 infections.
China: Fastest-growing Country-level Market
Based on geography, China is slated to record the highest growth rate during the forecast period. The rising prevalence of infectious & chronic diseases, the growing aging population, and the increasing adoption of self-testing mainly drive the growth of this market. Healthcare spending in China has shown year-on-year growth. According to The World Bank Group, the health expenditure (% of GDP) increased from 4.94% in 2015 to 5.59% in 2020.
Key Players
The key players profiled in this market study are Abbott Laboratories (U.S.), Siemens Healthineers AG (Germany), QuidelOrtho Corporation (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Danaher Corporation (U.S.), Becton, Dickinson and Company (U.S.), Trinity Biotech plc (Ireland), Werfen (Spain), Nova Biomedical (U.S.), Sekisui Diagnostics, LLC. (U.S.), Thermo Fisher Scientific Inc. (U.S.), and bioMérieux S.A. (France).
Scope of the Report:
Asia-Pacific Point-of-Care Diagnostics Market Assessment, by Offering
Asia-Pacific Point-of-Care Diagnostics Market Assessment, by Platform
Asia-Pacific Point-of-Care Diagnostics Market Assessment, by Application
Asia-Pacific Point-of-Care Diagnostics Market Assessment, by Sample Type
Asia-Pacific Point-of-Care Diagnostics Market Assessment, by End User
Asia-Pacific Point-of-Care Diagnostics Market Assessment, by Geography
Key questions answered in the report:
The Asia-Pacific Point-of-Care market study covers the market sizes & forecasts for types of point-of-care tests used in hospitals, home care/self-testing, physician offices & ambulatory care settings, diagnostics laboratories. The report includes the value analysis of various segments of the Asia-Pacific Point-of-Care at the country/region level.
The Asia-Pacific Point-of-Care market is projected to reach $11.80 billion by 2030, at a CAGR of 7.5% during the forecast period.
Among the platforms included in the report, in 2024, the lateral flow assays segment is estimated to hold the major share due to factors such as their long shelf life, higher portability, one-step process, and lower costs compared to laboratory-based tests.
Among the applications included in the report, the tumor/cancer markers testing segment is expected to grow with the highest CAGR. Factors driving the growth of this segment are the increasing incidence of cancer and developments in cancer marker testing.
Among the sample types included in the report, the blood sample segment is expected to grow with the highest CAGR due to the availability of a wide range of tests that can be conducted using blood samples.
Among the end user included in the report, the hospitals segment is expected to grow with the highest CAGR due to well-established infrastructure, an increasing patient inflow, and technological advancements.
The growth of this market is driven by the increasing prevalence of chronic diseases, innovations in POC tests, and a lack of skilled technicians for performing lab tests. Furthermore, healthcare professionals’ increasing preference for PoC tests over lab tests will offer significant market growth opportunities.
The key players operating in the Asia-Pacific Point-of-Care market are Abbott Laboratories (U.S.), Siemens Healthineers AG (Germany), QuidelOrtho Corporation (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Danaher Corporation (U.S.), Becton, Dickinson and Company (U.S.), Trinity Biotech plc (Ireland), Werfen (Spain), Nova Biomedical (U.S.), Sekisui Diagnostics, LLC. (U.S.), Thermo Fisher Scientific Inc. (U.S.), and bioMérieux S.A. (France).
China is projected to offer significant growth opportunities for vendors operating in this market due to the continuous improvement in the healthcare infrastructure and growing government investment in this sector.

























Published Date: Jul-2024
Published Date: Jul-2024
Published Date: Jul-2024
Published Date: Jul-2024
Published Date: Jul-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates