Resources
About Us
Asia-Pacific IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach - Forecast to 2032
Report ID: MRHC - 1041067 Pages: 230 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportIn vitro diagnostics (IVD) is focused on analyzing urine, blood, and other bodily fluid samples to provide diagnostic results. IVD tests help diagnose, monitor, screen, and assess susceptibility to diseases. These tests also aid in providing early and targeted treatments, reducing hospital stays, improving health outcomes, and minimizing treatment costs.
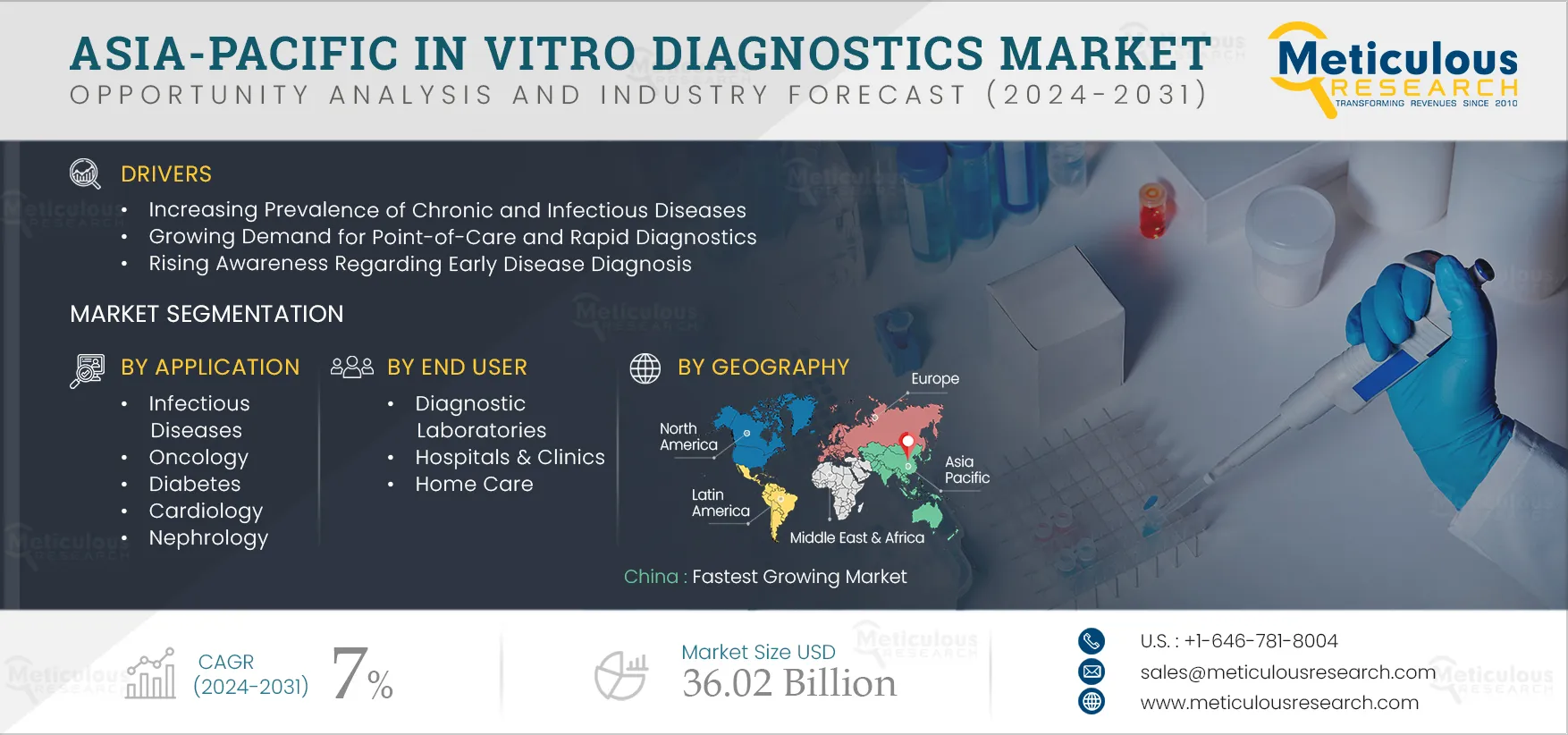
The growth of the Asia-Pacific IVD market is attributed to the rising prevalence of chronic and infectious diseases, the growing demand for point-of-care (PoC) and rapid diagnostics, rising awareness regarding early disease diagnosis, rising healthcare expenditures, and increasing funding for research activities. However, stringent technical requirements and regulatory processes for high/moderate-complexity tests and the variance in test results observed in rapid IVD tests restrain the growth of the IVD market. The increasing inclination toward personalized medicine and advancements in genomics and proteomics are expected to create growth opportunities for the players operating in this market. However, the concerns pertaining to false positive results in immunoassays and POC are a major challenge for market growth.
In recent years, there has been a continuous shift toward the decentralization of IVD testing, resulting in the proliferation of point-of-care (PoC) testing services and the development of rapid tests. Point-of-care (PoC) and rapid diagnostics offer benefits such as cost reduction, convenience for patients and clinicians, reduced waiting time for results, and improved testing quality. Traditional laboratory technologies require sophisticated infrastructure, expensive reagents, stable electrical power, long assay times, and skilled and trained staff. In developed countries, hospitals and large clinics have the necessary infrastructure and purchasing power to meet these requirements. However, in the APAC region, the adoption of PoC can be proven useful for the testing in resource-limited testing.
Additionally, key companies are developing various tests and instruments for rapid disease diagnosis. Some of the product’s approval and launch in recent years are as follows:
Click here to: Get Free Sample Pages of this Report
Chronic diseases are often associated with the elderly population due to the declining bodily functions and immunity among this population segment. Aging is associated with progressive deterioration in the structure and functioning of organs. The elderly population is more prone to various chronic diseases. According to the World Bank, the share of the elderly population (aged 65 and above) in Japan is among the highest in the world and is growing at a CAGR of 1.1% (from 2016 to 2021). In 2021, the share of the elderly population in Japan was estimated at 29.8% of the country’s total population (source: World Bank).
The prevalence of chronic diseases and conditions such as cancer, diabetes, arthritis, and heart disease is on the rise in Asia-Pacific countries. In 2021, 140.9 million people were living with diabetes in China, which is expected to reach 174.4 million by 2045 (Source: IDF). Similarly, in 2021, 74.2 million people were living with diabetes in India, which is expected to reach 124.9 million by 2045 (Source: IDF). As IVD tests are widely adopted for glucose monitoring, the prevalence of diabetes drives the adoption of IVD devices.
The growth in the geriatric population and social behaviours such as tobacco and alcohol consumption, physical inactivity, and unhealthy diets are major factors driving a steady increase in the number of people suffering from chronic diseases. The growth in the elderly population and the associated increase in the burden of chronic disease is increasing the demand for IVD testing in Asia-Pacific.
Based on offering, the Asia-Pacific IVD market is segmented into reagents and kits, instruments, and software & services. In 2025, the kits & reagents segment is expected to account for the largest share of the market. The continuous advancements in the development of reagents and kits, high adoption and recurring consumption of IVD kits, and wide availability of consumables support the largest share of the market. The increasing trend of self-testing and adoption of POC diagnostic testing is contributing to the largest share of this segment.
Based on technology, the Asia-Pacific IVD market is segmented into immunoassay/immunochemistry, biochemistry/clinical chemistry, molecular diagnostics, point-of-care (POC) diagnostics, whole blood glucose monitoring, microbiology, hematology, coagulation/hemostasis, urinalysis, and other technologies. In 2025, the molecular diagnostics segment was expected to account for the largest share of the market. The growing prevalence of infectious diseases is also increasing the demand for molecular diagnostics. For instance, In Australia, between 2020 to 2021, there were more than 324,000 hospitalizations for infectious diseases. The most common causes of infectious disease hospitalization were lower respiratory tract infections, including pneumonia and bronchitis. (Source: Australian Institute of Health and Welfare). Additionally, technological innovations and the advantages of molecular diagnostics tests, such as cost-effectiveness, rapid results, and high sensitivity and specificity over laboratory tests.
Based on application, the Asia-Pacific in vitro diagnostics (IVD) market is segmented into infectious diseases, oncology, diabetes, cardiology, nephrology, autoimmune disorders, and other applications. The cardiology segment is expected to register the highest CAGR during the forecast period. Cardiovascular diseases (CVD) remain the leading cause of death globally. IVD testing is used for biomarker detection in cardiology. The early-stage biomarkers of CVD can potentially save many lives and help alleviate the global burden of CVD. According to the American Heart Association, in 2020 highest mortality rates due to cardiovascular diseases were reported in Central Asia and Eastern Asia-Pacific. Thus, the high prevalence of cardiovascular diseases is expected to drive the demand for IVD testing in Asia-Pacific.
Based on diagnostic approach, the Asia-Pacific IVD market is segmented into laboratory testing, OTC/self-testing, and point-of-care testing. In 2025, the laboratory testing segment is expected to account for the largest share of the market. The large market share of this segment is primarily attributed to its higher accuracy and reliability, lower costs, and availability of several IVD tests in laboratory settings. The laboratories usually include a quality assurance program and address pre- and post-analytical concerns, improving and maintaining the accuracy of laboratory testing.
Based on end user, the Asia-Pacific in vitro diagnostics (IVD) market is segmented into diagnostic laboratories, hospitals & clinics, home healthcare, and other end users, which include nursing homes, academic & research institutes, ambulatory care centers, and transfusion laboratories. The hospitals & clinics segment is expected to register the highest CAGR during the forecast period. The highest CAGR of this segment is attributed to the increasing number of hospitalizations due to various diseases requiring diagnosis and the rise in the number of hospitals and clinics in the region, leading to growth in the utilization of diagnostic products.
China is expected to register the highest CAGR during the forecast period. The highest CAGR of this regional market is attributed to the rising prevalence of infectious & chronic diseases, the growing aging population, and the increasing adoption of self-testing. Additionally, China is one of the world's most populous countries, with a population of over 1.41 billion in 2021 (Source: World Bank). There is an increasing burden on the country's healthcare system due to the growing elderly population, high prevalence of cancer, and increasing sedentary lifestyle. According to the International Diabetes Federation (IDF), China has the highest number of people with diabetes globally. In 2021, 140.9 million people were living with diabetes in China, which is expected to reach 174.4 million by 2045 (Source: IDF). With the increasing number of chronic conditions, the demand for IVD testing is increasing in the country.
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments adopted by leading market players in the industry over the years. The key players operating in the Asia-Pacific IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), Sysmex Corporation (Japan), bioMérieux SA (France), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Seegene Inc. (South Korea), Illumina, Inc. (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), QuidelOrtho Corporation (U.S.), and Agilent Technologies Inc. (U.S.).
Report Summary:
|
Particular |
Details |
|
Number of Pages |
~230 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2024 |
|
CAGR |
7% |
|
Estimated Market Size (Value) |
$36.02 billion by 2032 |
|
Segments Covered |
by Offering
by Technology
by Application
by Diagnostic Approach
by End User
by Country/Region
|
|
Countries Covered |
Asia-Pacific (China, Japan, India, Australia, South Korea, Thailand, Singapore, Indonesia, RoAPAC) |
|
Key Companies |
Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), Sysmex Corporation (Japan), bioMérieux SA (France), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Seegene Inc. (South Korea), Illumina, Inc. (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), QuidelOrtho Corporation (U.S.), and Agilent Technologies Inc. (U.S.). |
Key questions answered in the report:
The Asia-Pacific IVD market is segmented based on product & solution (reagents & kits, systems, and software & services) and a wide range of technologies (immunoassay/immunochemistry, biochemistry/clinical chemistry, molecular diagnostics, microbiology, hematology, coagulation/hemostasis, urinalysis, and other technologies) offered by key companies to various end users, such as diagnostic laboratories, hospitals & clinics, home healthcare, and other end users. The Asia-Pacific IVD market studied in this report involves the value analysis of various segments and sub-segments of in-vitro diagnostics at regional and country levels.
The Asia-Pacific IVD market is projected to reach $36.02 billion by 2032, at a CAGR of 7.0% during the forecast period.
The kits & reagents segment is estimated to account for the largest share of the Asia-Pacific IVD market in 2025. Factors such as the high burden of diseases, the high adoption of rapid diagnostic test kits, and the presence of initiatives supporting early disease diagnosis are responsible for the largest market share.
Based on the technology, the molecular diagnostics segment is projected to create more traction owing to the technological innovations and the advantages of rapid immunoassay tests, such as cost-effectiveness, rapid results, and high sensitivity & specificity over laboratory tests.
Based on application, the cardiology segment is projected to create more traction during the forecast period due to the growing burden of cardiovascular diseases and rising demand for early diagnosis.
Based on end user, the hospitals & clinics segment is projected to create more traction during the forecast period due to a large number of hospitalizations due to various diseases, the rising geriatric population, increasing healthcare access & expenditure, and the rising prevalence of healthcare-associated infections (HAIs).
The growth of the Asia-Pacific IVD market is attributed to the rising prevalence of chronic and infectious diseases, the growing demand for point-of-care (PoC) and rapid diagnostics, rising awareness regarding early disease diagnosis, rising healthcare expenditures, and increasing funding for research activities. The increasing inclination toward personalized medicine and advancements in genomics and proteomics are expected to create growth opportunities for the players operating in this market.
The key players operating in the Asia-Pacific IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), Sysmex Corporation (Japan), bioMérieux SA (France), Bio-Rad Laboratories, Inc. (U.S.), Danaher Corporation (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Seegene Inc. (South Korea), Illumina, Inc. (U.S.), QIAGEN N.V. (Netherlands), Shenzhen Mindray Bio-Medical Electronics Co., Ltd (China), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), QuidelOrtho Corporation (U.S.), and Agilent Technologies Inc. (U.S.).
China is expected to offer significant growth opportunities owing to the government initiatives to improve the healthcare infrastructure in the country, the rising prevalence of infectious diseases, and the growth in the geriatric population.
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates