1. Introduction
1.1. Market Definition
1.2. Market Ecosystem
1.3. Currency
1.4. Key Stakeholders
2. Research Methodology
2.1. Research Process
2.2. Data Collection & Validation
2.2.1. Secondary Research
2.2.2. Primary Research
2.3. Market Assessment
2.3.1. Market Size Estimation
2.3.1.1. Bottom-Up Approach
2.3.1.2. Top-Down Approach
2.3.1.3. Growth Forecast
2.3.2. Market Share Analysis
2.4. Assumptions For The Study
2.5. Limitations For The Study
3. Executive Summary
4. Market insights
4.1. Introduction
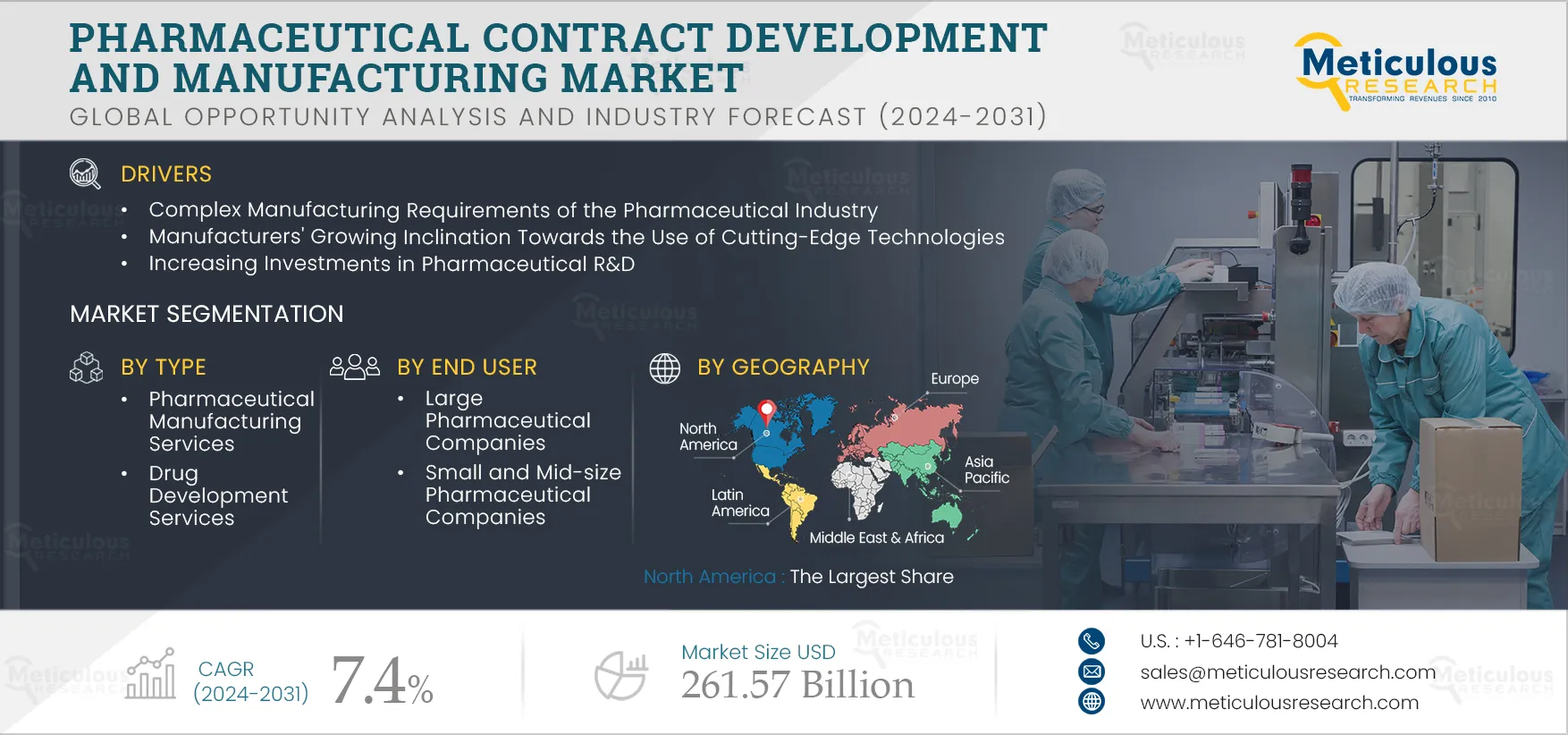
4.2. Drivers
4.2.1. Complex Manufacturing Requirements of the Pharmaceutical industry and Manufacturers' Growing inclination
Toward the Use of Cutting-Edge Technologies
4.2.2. Rising Demand for Generic Medicines and Biologics
4.2.3. Patent Expiration
4.2.4. Increasing Investments in Pharmaceutical R&D
4.2.5. Outbreak of the COVID-19 Pandemic
4.3. Challenges
4.3.1. Shortage of Skilled Professionals
4.3.2. Introduction of Serialization
4.4. Opportunities
4.4.1. Growing Demand for Cell Therapies, Gene Therapies, and Personalized Medicines
4.4.2. Growth in High Potency Active Pharmaceutical Ingredients (HPAPI) and Antibody-drug Conjugates (ADC) Markets
4.5. Industry Trends
4.5.1. Virtual Business Models
4.5.2. Growing Consolidation Among CDMO Market Players
5. Pharmaceutical Contract Development and Manufacturing Services Market, by Type
5.1. Introduction
5.2. Pharmaceutical Manufacturing Services
5.2.1. Active Pharmaceutical ingredient (API) Manufacturing Services
5.2.2. Finished Dosage Forms (FDF) Manufacturing Services
5.2.2.1. Parenteral/Injectable Manufacturing Services
5.2.2.2. Tablet Manufacturing Services
5.2.2.3. Capsule Manufacturing Services
5.2.2.4. Oral Liquid Manufacturing Services
5.2.2.5. Other Formulations
5.3. Drug Development Services
5.4. Biologics Manufacturing Services
5.4.1. Active Pharmaceutical Manufacturing Services (API) Manufacturing Services
5.4.2. Finished Dosage Form Manufacturing (FDF) Services
6. Pharmaceutical Contract Development and Manufacturing Market, by End User
6.1. Introduction
6.2. Large Pharmaceutical Companies
6.3. Small & Mid-Size Pharmaceutical Companies
6.4. Generic Pharmaceutical Companies
7. Pharmaceutical Contract Development and Manufacturing Market, By Geography
7.1. Introduction
7.2. North America
7.2.1. U.S.
7.2.2. Canada
7.3. Europe
7.3.1. Germany
7.3.2. U.K.
7.3.3. France
7.3.4. Italy
7.3.5. Spain
7.3.6. Rest of Europe (RoE)
7.4. Asia-Pacific
7.4.1. China
7.4.2. India
7.4.3. Japan
7.4.4. Rest of Asia-Pacific (ROAPAC)
7.5. Latin America
7.5.1. Brazil
7.5.2. Mexico
7.5.3. Rest of Latin America
7.6. Middle East & Africa
8. Competitive Landscape
8.1. Introduction
8.2. Key Growth Strategies
8.3. Competitive Benchmarking
8.4. Market Share Analysis (2021) – TOP 19 Players
8.4.1. Lonza Group Ltd. (Switzerland)
8.4.2. Catalent INC. (U.S.)
8.4.3. Thermo Fisher Scientific INC. (U.S.)
8.4.4. Samsung Biologics Co., Ltd. (South Korea)
8.4.5. Recipharm AB (Sweden)
9. Company Profiles (Business Overview, CDMO Facilities, Financial Overview, Service Portfolio, Strategic Developments)
9.1. Cambrex Corporation
9.2. Samsung Biologics Co., Ltd.
9.3. Siegfried Holdings AG
9.4. FUJIFILM Diosynth Biotechnologies
9.5. Aenova Group
9.6. Wuxi Biologics, Inc.
9.7. Aurobindo Pharma, Ltd.
9.8. Vetter Pharma international GmbH
9.9. Thermo Fisher Scientific, Inc.
9.10. Catalent, Inc
9.11. Recipharm AB
9.12. Almac Group
9.13. Lonza Group Ltd.
9.14. Piramal Enterprises Limited
9.15. Fabbrica Italiana Sintetici S.p.A.
9.16. Fareva SA
9.17. C.H. Boehringer Sohn Ag & Co. KG.
9.18. Jubilant Pharmova Limited
9.19. Curia Global, Inc.
10. Appendix
10.1. Questionnaire
10.2. Available Customization
List of Tables
Table 1 Global Pharmaceutical Contract Development and Manufacturing Market Drivers: Impact Analysis (2022–2029)
Table 2 Patent Expiration Of Top 15 Drugs
Table 3 Pharmaceutical R&D Spending by Top 10 Companies
Table 4 Expansions by CDMOs in HPAPI Development and Manufacturing (2020–2022)
Table 5 Consolidation in the Pharmaceutical Contract Development and Manufacturing Market
Table 6 Pharmaceutical Contract Development & Manufacturing: Expansions
Table 7 Global Pharmaceutical Contract Development and Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 8 Global Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 9 Pharmaceutical Contract Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 10 API Contract Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 11 Global FDF Contract Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 12 FDF Contract Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 13 Number Of Parenteral Drugs Approved in Relation To Total New Molecular Entity (NME) Approved (2015–2020)
Table 14 Parenteral/Injectable Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 15 Tablet Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 16 Capsule Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 17 Oral Liquid Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 18 Other Formulations Market Size, by Region/Country, 2020–2029 (USD Million)
Table 19 Contract Drug Development Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 20 Global Biologics Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 21 Biologics Contract Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 22 API Contract Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 23 FDF Contract Manufacturing Services Market Size, by Region/Country, 2020–2029 (USD Million)
Table 24 Global Pharmaceutical Contract Development and Manufacturing Market, by End User, 2020–2029 (USD Million)
Table 25 Pharmaceutical Contract Development and Manufacturing Market For Large Pharmaceutical Companies, by
Country/Region, 2020–2029 (USD Million)
Table 26 Pharmaceutical Contract Development and Manufacturing Market For Small & Mid-Size Pharmaceutical Companies,
by Country/Region, 2020–2029 (USD Million)
Table 27 Pharmaceutical Contract Development and Manufacturing Market For Generic Pharmaceutical Companies, by Country/Region, 2020–2029 (USD Million)
Table 28 Global Pharmaceutical Contract Development and Manufacturing Market Size, by Country/REGION, 2020–2029 (USD Million)
Table 29 North America: Pharmaceutical Contract Development and Manufacturing Market Size, by Country, 2020–2029 (USD Million)
Table 30 North America: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 31 North America: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 32 North America: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 33 North America: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 34 North America: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 35 Key Developments by Companies With Respect to COVID-19 Vaccine
Table 36 U.S.: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 37 U.S.: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 38 U.S.: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 39 U.S.: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 40 U.S.: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 41 Canada: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 42 Canada: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 43 Canada: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 44 Canada: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 45 Canada: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 46 Europe: Pharmaceutical Contract Development and Manufacturing Market Size, by Country/Region, 2020–2029 (USD Million)
Table 47 Europe: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 48 Europe: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)2
Table 49 Europe: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 50 Europe: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 51 Europe: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 52 Germany: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 53 Germany: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 54 Germany: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 55 Germany: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 56 Germany: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 57 Key Developments to Support Drug Development Services in the U.K.
Table 58 U.K.: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 59 U.K.: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 60 U.K.: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 61 U.K.: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 62 U.K.: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 63 France: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 64 France: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 65 France: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 66 France: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 67 France: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 68 Italy: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 69 Italy: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 70 Italy: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 71 Italy: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 72 Italy: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 73 Spain: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 74 Spain: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 75 Spain: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 76 Spain: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 99 Spain: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 100 Rest of Europe: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 101 Rest of Europe: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 102 Rest of Europe: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 104 Rest of Europe: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 105 Rest of Europe: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 106 Asia-Pacific: Pharmaceutical Contract Development and Manufacturing Market Size, by Country/Region, 2020–2029 (USD Million)
Table 107 Asia-Pacific: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 108 Asia-Pacific: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 110 Asia-Pacific: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 112 Asia-Pacific: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 113 Asia-Pacific: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 114 China: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 115 China: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 116 China: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 117 China: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 118 China: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 119 India: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 120 India: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 121 India: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 122 India: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 123 India: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 124 Japan: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 125 Japan: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 126 Japan: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 127 Japan: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 128 Japan: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 129 RoAPAC: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 130 RoAPAC: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 131 RoAPAC: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 132 RoAPAC: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 133 RoAPAC: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 134 Latin America: Pharmaceutical Contract Development and Manufacturing Market Size, by Country/Region, 2020–2029 (USD Million)
Table 135 Latin America: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 136 Latin America: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 137 Latin America: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 138 Latin America: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 139 Latin America: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 140 Brazil: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 141 Brazil: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 142 Brazil: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 143 Brazil: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 144 Brazil: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 145 Mexico: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 146 Mexico: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)6
Table 147 Mexico: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 148 Mexico: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 149 Mexico: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 150 RoLATAM: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 151 RoLATAM: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 152 RoLATAM: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 153 RoLATAM: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 154 RoLATAM: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 155 Middle East & Africa: Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2020–2029 (USD Million)
Table 156 Middle East & Africa: Pharmaceutical Contract Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 157 Middle East & Africa: Pharmaceutical FDF Manufacturing Services Market Size, by Formulation, 2020–2029 (USD Million)
Table 158 Middle East & Africa: Biologics Manufacturing Services Market Size, by Type, 2020–2029 (USD Million)
Table 159 Middle East & Africa: Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2020–2029 (USD Million)
Table 160 Recent Developments, by Company, 2019–2022
List of Figures
Figure 1 Research Process
Figure 2 Key Secondary Sources
Figure 3 Primary Research Techniques
Figure 4 Key Executives interviewed
Figure 5 Breakdown Of Primary interviews (Supply-Side & Demand-Side)
Figure 6 Market Size Estimation
Figure 7 Global Pharmaceutical Contract Development and Manufacturing Market Size, by Service, 2022 VS. 2029 (USD Million)
Figure 8 Global Pharmaceutical Fdf Manufacturing Services Market Size, by Formulation, 2022 VS. 2029 (USD Million)
Figure 9 Global Pharmaceutical Contract Development and Manufacturing Market Size, by End User, 2022 VS. 2029 (USD Million)
Figure 10 Pharmaceutical Contract Development and Manufacturing Market Size, by Geography
Figure 11 Market Dynamics
Figure 12 Worldwide Sales for Generics (2018–2026)
Figure 13 Global Spending on Pharmaceutical Research and Development (USD Billion)
Figure 14 Global Pharmaceutical Contract Development and Manufacturing Services Market Size, by Type, 2022–2029 (USD Million)
Figure 15 Worldwide Generic Drug Sales (2018–2026)
Figure 16 Global Pharmaceutical Contract Development and Manufacturing Market, by End User, 2022–2029 (USD Million)
Figure 17 Global Pharmaceutical Contract Development and Manufacturing Market, by Region, 2022–2029 (USD Million)
Figure 18 North America: Pharmaceutical Contract Development and Manufacturing Market Snapshot
Figure 19 Europe: Pharmaceutical Contract Development and Manufacturing Market Snapshot
Figure 20 Biosimilar Consumption in Europe, 2018
Figure 21 Asia-Pacific: Pharmaceutical Contract Development and Manufacturing Market Snapshot
Figure 22 Latin America: Pharmaceutical Contract Development and Manufacturing Market Snapshot
Figure 23 Key Growth Strategies Adopted by Leading Players, 2019–2022
Figure 24 Pharmaceutical Contract Development and Manufacturing Market: Competitive Benchmarking, by Service
Figure 25 Market Share Analysis: Pharmaceutical Contract Development and Manufacturing Services Industry, 2021
Figure 26 Samsung Biologics Co., Ltd.: Financial Overview (2021)
Figure 27 Siegfried Holdings AG.: Financial Overview (2021)
Figure 28 FUJIFILM Holdings Corporation: Financial Overview (2020)
Figure 29 Aenova Group: Financial Overview (2020)
Figure 30 Wuxi Biologics Inc: Financial Overview (2021)
Figure 31 Aurobindo Pharma Ltd: Financial Overview (2020)
Figure 32 Thermo Fisher Scientific, Inc.: Financial Overview (2021)
Figure 33 Catalent, Inc: Financial Overview (2021)
Figure 34 Recipharm AB: Financial Overview (2020)
Figure 35 Lonza Group Ltd: Financial Overview (2021)
Figure 36 Piramal Enterprises Limited: Financial Overview (2020)
Figure 37 C.H. Boehringer Sohn Ag & Co. KG.: Financial Overview (2021)
Figure 38 Jubilant Pharmova Limited: Financial Overview (2020)